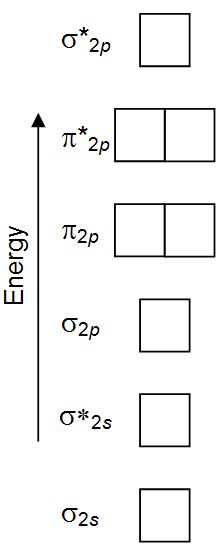
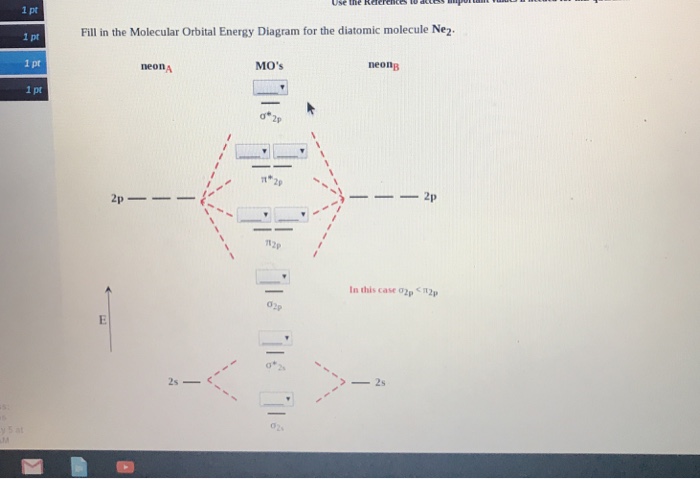
38 molecular orbital diagram for ne2
Figure 9.7. 3: Molecular Orbital Energy-Level Diagrams for Diatomic Molecules with Only 1 s Atomic Orbitals. (a) The H 2+ ion, (b) the He 2+ ion, and (c) the He 2 molecule are shown here. Figure 9.7. 3 a shows the energy-level diagram for the H 2+ ion, which contains two protons and only one electron. For Ne2, construct three molecular orbital diagrams, one each for the neutral molecule, the +1 cation, and the -1 anion. Give each MO an appropriate label. Determine the electron configuration and bond order for each, and rank the three species in order of increasing bond order. Rationalize the trend in bond order in terms of bond strength.
Molecular Orbital Diagram for Nitrogen Gas (+1 ion) (N2(+)).Fill from the bottom up, with 9 valence electrons total.Bonding Order is 2.5, so it is unstable, ...
Molecular orbital diagram for ne2
After reading the theory part draw the MO diagrams for the ... H2, B2, C2, N2, O2, Ne2, F2 ... Ne2. F2 electron configuration. N. OF. OCCUPIED. MO.13 pages From Molecular Orbital Diagram, which is most stable? A. F2 2-B. Ne2 2+ C. O2 2+ D. F2 E. F2 2+ C. O2 2+ Choose the compound below that should have the highest melting point according to the ionic bonding model. A. CaS B. NaCl C. RbI D. MgO E. AlN. E. AlN. Molecular Orbital Diagram For Ne2. Mar 4, Find an answer to your question Draw and explain the molecular orbital diagram of Ne2. On the basis of molecular orbital diagram, explain. According to Molecular Orbital theory, only those molecule can exists which have net positive bond order while the molecules with negative or.
Molecular orbital diagram for ne2. Problem: Determine the bond order from the molecular orbital diagram of Ne2. Does the bond order calculated agree with what you would draw for Lewis structures ...1 answer · Top answer: We are asked to determine the bond order from the molecular orbital diagram of Ne2 and to check whether the calculated bond order agrees with the ... Even rather simple molecular orbital (MO) theory can be used to predict from the bottom of the diagram because this is how MO diagrams are constructed, from N2, O2, F2, Ne2 the complexity of the molecular orbitals develop in two ways. Page 1. MO Diagrams for Elements Li2 through Ne2. (Don't memorize.) Li2 through N2. O2 through Ne2. Molecular Orbital Diagram For Ne2. A molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals lcao method in particular. The orbital correlation diagram in predicts the same thing two electrons ... There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc).One is for the elements up to Nitrogen. The other is for AFTER nitrogen (start...
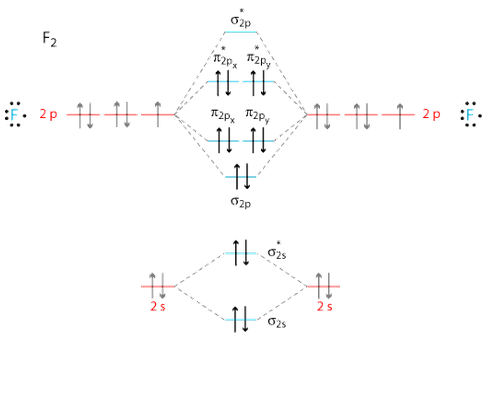
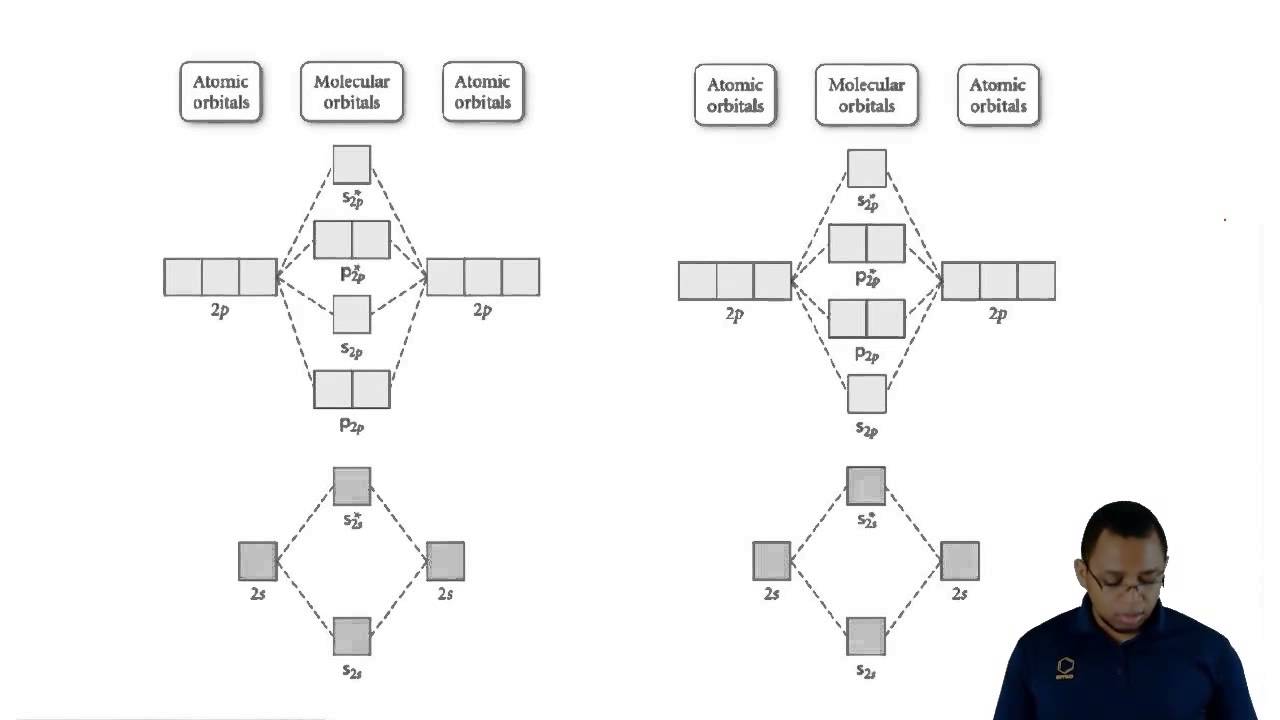
The molecular orbital theory (MO) has been introduced for the diatomic hydrogen molecules. The same method can be applied to other diatomic molecules, but involving more than the 1s atomic orbitals. For the second period elements, the 2s and 2p orbitals are important for MO considerations. A linear combination of properly oriented atomic orbitals for the formation of sigma s and pi p bonds. Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ... Molecular Orbital Diagram Molecular orbital theory helps Chapter 9 Section 6 Molecular orbital theory helps you predict several important properties of the substance. Bond order (bond length and bond strength). Bond enthalpy (bond energy) Dr. A. Al-Saadi 22 Magnetic properties. MO diagram of O 2 Answer: First let me make it clear that Ne, which is Neon, is a noble gas. Noble gases rarely form compounds and they don't exist as molecules in their pure form. So, Neon, as a gas, exists as only Ne and not as Ne2 (Oxygen, Hydrogen, Nitrogen are some of the many gases that exist as molecules in...
Molecular orbital diagram for nitrogen gas (N2)Use aufbau and Hund to fill with 10 valence electronsYou get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2).Bond Or... In Ne2 ion, the electronic configuration of Ne is Ne 1s2s22p6 Calculate the total number 2s 2s 1s ls Ne2 Bond order specifies the strength of the bond. 0:21 Molecular Orbital Diagram of Oxygen Molecule3:30 Molecular Orbital Diagram of Florine Molecule5:25 Molecular Orbital Diagram of Neon MoleculeSo as we d... the pi(2p) bonding orbitals are LOWER than the sigma(2p) bonding orbitals.N2(2-) has a bonding order of 2, which predicts that there will be a stable double ...
23 May 2020 — If the M.O. diagram is drawn it is seen that no. Of bonding orbitals = no. Of antibonding orbitals. Bond order = no. Of bonds = B.Os ...4 answers · 5 votes: Molecular orbital confuguration of Ne2 is σ1s²σ*1s²σ2s²σ*2s²σ2Pz²π2Px²π2Py²π*2Px²π ...Is NE2 2+ paramagnetic or diamagnetic? - Quora1 answer27 Nov 2020What is the molecular orbital diagram of O2 and F2 ...6 answers12 Mar 2017By using molecular orbital theory, why doesn't the Ne2 ...4 answers9 Aug 2018Why is the molecular orbital diagram for O₂ ...2 answers1 Aug 2019More results from www.quora.com
Using The Arrows Given Below Complete The Molecular Orbital Occupancy For Ne2 Do Not Leave Any Homeworklib
An advanced molecular orbital diagram of beh2 beryllium hydride for the inorganic or physical chemistry student. Molecular orbitals and walsh diagram. Walsh correlation diagram is a plot of molecular orbital energy as a function of some systematic change in molecular geometry. Be has 2s and 2p orbitals and it is in the middle.
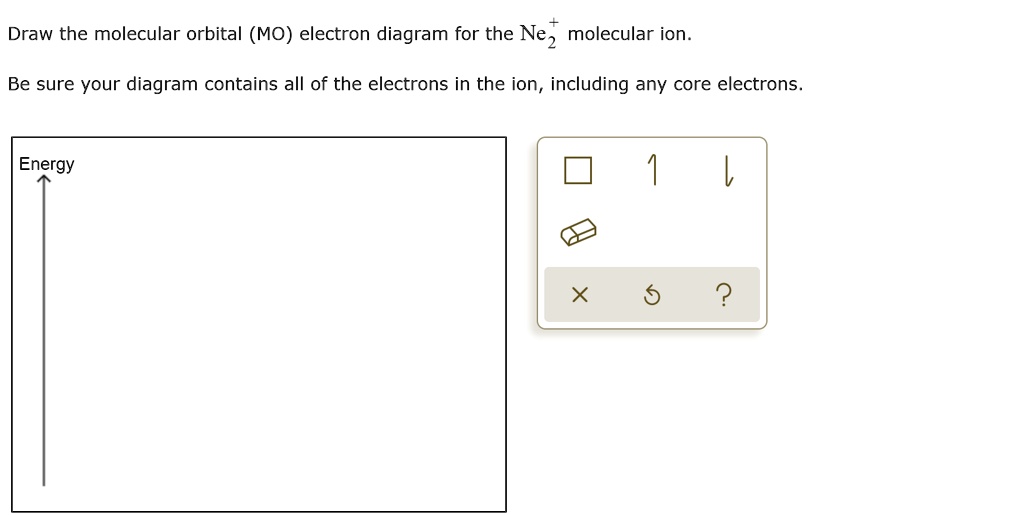
Solved Draw The Molecular Orbital Mo Electron Diagram For The Ne2 Molecular Ion Be Sure Your Diagram Contains All Of The Electrons In The Ion Including Any Core Electrons Energy 2
sorry about that not being a molecular orbital diagram i saw orbital and immediately thought electron configuration to save confusion, could. There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc) . One is for the elements up to Nitrogen. The other is for AFTER nitrogen.
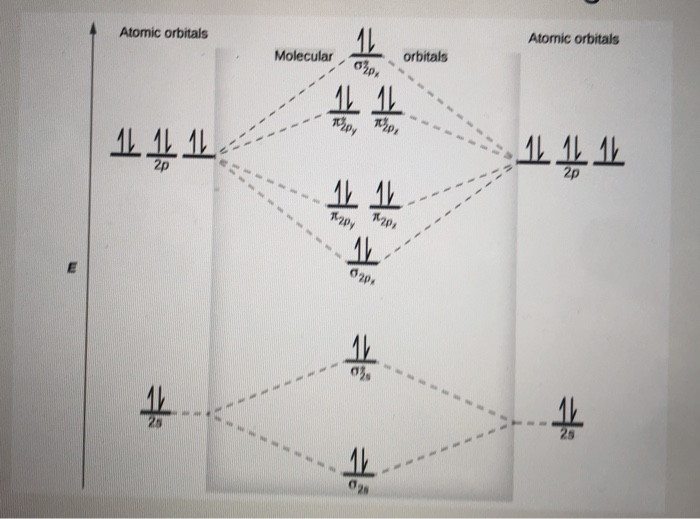
26 Jan 2020 — With the help of molecular orbital diagram show that Ne2 cannot exist as stable molecule. (Atomic number of Ne = 10). chemical bonding ...1 answer · Top answer: Ne2 (20) = σ1s2 σ*1s2, σ2s2 σ*2s2, σpx2 π 2py2 2π* 2py2π*2py2 2pz2 σ* 2px2 B.O = 1/2 (10 - 10) = 0 Ne2 cannot exist because its bond order is zero.

Use The Molecular Orbital Energy Level Diagram To Show That N 2 Would Be Expected To Have A Triple Bond F 2 A Single Bond And Ne 2 No Sarthaks Econnect Largest
Even rather simple molecular orbital (MO) theory can be used to predict from the bottom of the diagram because this is how MO diagrams are constructed, from N2, O2, F2, Ne2 the complexity of the molecular orbitals develop in two ways.Draw the molecular orbital diagram for Ne 2 + and determine if the bond between the two atoms will be stable.
Exercise 3.3.4. 3. Construct a qualitative molecular orbital diagram for chlorine, Cl 2. Compare the bond order to that seen in the Lewis structure (remember that an electron in an antibonding orbital cancels the stabilization due to bonding of an electron in a bonding orbital). Answer.

Use The Molecular Orbital Energy Level Diagram To Show That Cbse Class 11 Chemistry Learn Cbse Forum
4 Mar 2018 — Molecular orbital diagram of · Neon atom has 10 electrons and its electronic configuration is . When molecule is considered, it has two neon ...2 answers · 18 votes: Bond order of Ne2 = (10-10) =0 So Ne2 is unstable ;Ne2 cannot exist
Molecular Orbital Diagrams simplified. Megan Lim. Oct 26, 2016 · 3 min read. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding ...

Energy Level Diagram For Molecular Orbitals Chemical Bonding And Molecular Structure Chemistry Class 11
Use the molecular orbital energy level diagram to show that N2 would be expected to have a triple bond, F2, a single bond and Ne2, no bond. · Solution.1 answer · Top answer: Formation of N2 molecule: Electronic Configuration, σ 1s^2<σ *1s^2<σ 2s^2<σ *2s^2<[pi 2px^2 = pi 2px^2]<<σ 2pz^2 Bond order = (Nb - Na)/2 = (10 - ...
Answer (1 of 2): Here is the MO diagram for O₂: Whilst this is the MO diagram for N₂: If we compare such diagrams for the diatomic molecules on the Second Period (Li₂, Be₂, B₂, C₂, N₂, O₂, and F₂), the resulting pattern looks like this: When it comes to O₂ and N₂, I think there are two things ...
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
A molecular orbital explicitly describes the spatial distribution of a single Energy Level Diagrams He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. According to the molecular orbital theory, in a supposed He2 molecule, both the if we draw its MOT DIAGRAM, 2 e's enter the Bonding molecular Orbital and 2 .
Answer (1 of 4): Neon is denoted by Ne which is noble gas. Noble gases are non-reactive by nature.The outermost shell in Ne element is fully filled with electrons where the electron is 10 in number. This means that will have 20 electrons. This is why it does not need to react with any other atom ...
Molecular Orbital Diagram For Ne2. Mar 4, Find an answer to your question Draw and explain the molecular orbital diagram of Ne2. On the basis of molecular orbital diagram, explain. According to Molecular Orbital theory, only those molecule can exists which have net positive bond order while the molecules with negative or.
From Molecular Orbital Diagram, which is most stable? A. F2 2-B. Ne2 2+ C. O2 2+ D. F2 E. F2 2+ C. O2 2+ Choose the compound below that should have the highest melting point according to the ionic bonding model. A. CaS B. NaCl C. RbI D. MgO E. AlN. E. AlN.
After reading the theory part draw the MO diagrams for the ... H2, B2, C2, N2, O2, Ne2, F2 ... Ne2. F2 electron configuration. N. OF. OCCUPIED. MO.13 pages

Draw The Molecular Orbital Diagram Of O2 And Calculate The Bond Order Is O2 Diamagnetic Or Paramagnetic Explain Your Answer Study Com
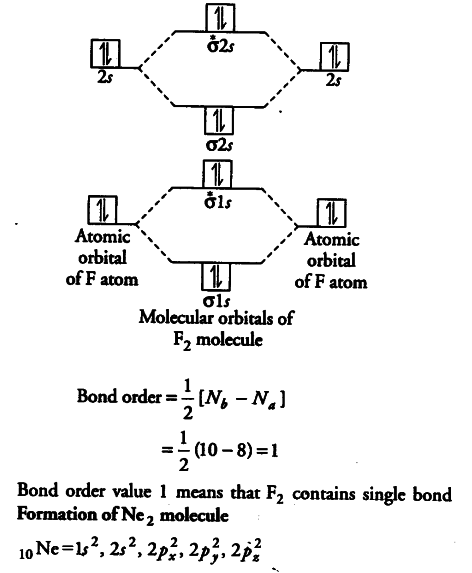
Use The Molecular Orbital Energy Level Diagram To Show That Cbse Class 11 Chemistry Learn Cbse Forum
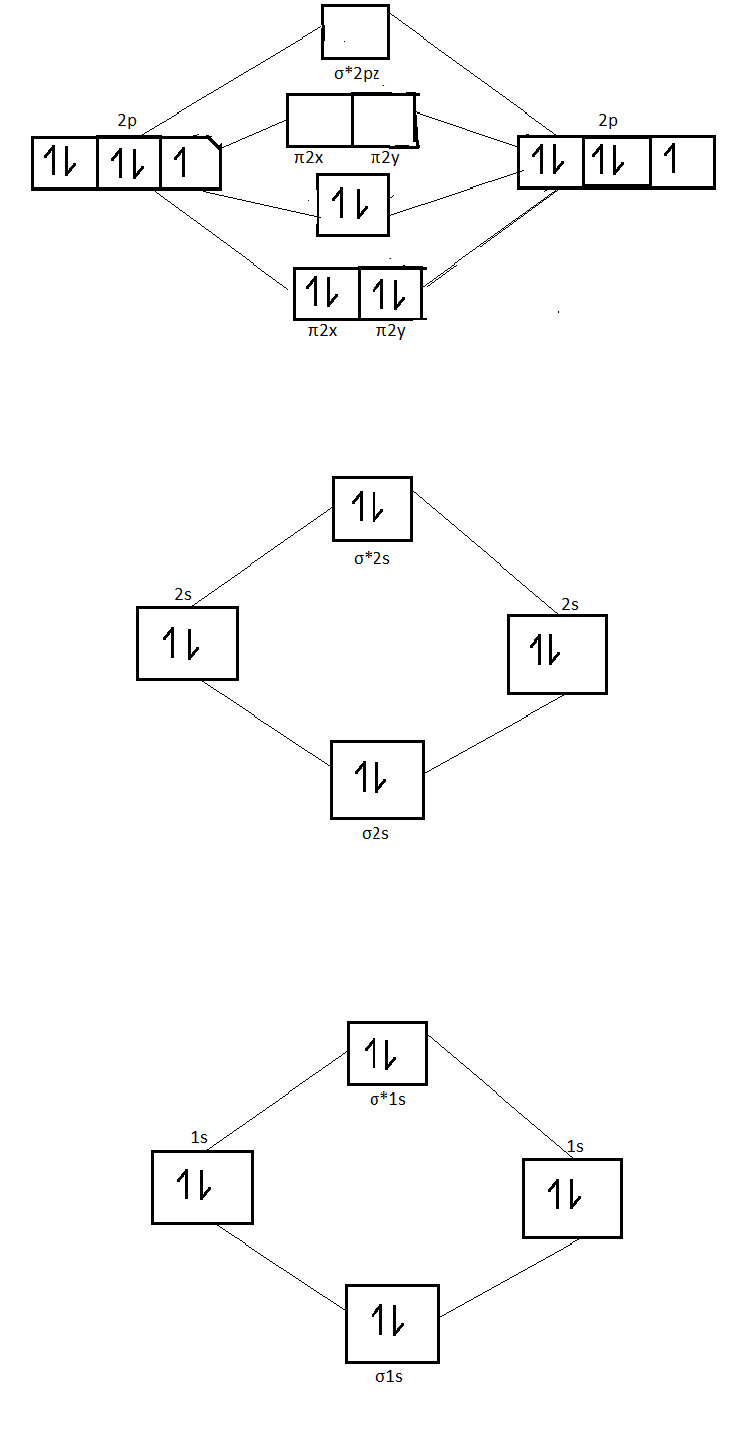













Comments
Post a Comment