41 sulfur atom diagram
Example: In N a 2 O, the oxidation number of O is − 2. In M g 0, the oxidation number of O is − 2. Peroxides: In peroxides, the oxidation number of oxygen is − 1. Examples, H 2 O 2, N a 2 O 2. In compounds with fluorine, the oxidation number of oxygen is + 2. Examples F 2 O or O F 2, etc. Amorphous or "plastic" sulfur is obtained by fast cooling of the crystalline form. X-ray studies indicate that amorphous sulfur may have a helical structure ...
SOBr2. The central atom is sulfur. There are 26 electrons to account for. Each bromine will have an octet, with three lone pairs. Sulfur has one lone pair, and oxygen has two lone pairs.
Sulfur atom diagram
6.5. Hydrogen peroxide, H2O2, reacts with sulfur trioxide to form peroxomonosulfuric acid, H2SO5, in a Lewis acid-base reaction. H2O2 + SO3 = H2SO5. c) Identify the lewis acid and base. Can someone explain how they determine the acid and base in this problem. The structure of sulfurous acid shows that the sulfur atom is bonded to two hydroxyls and a single oxygen atom. The sulfur is directly bonded with the oxygen in the hydroxyl S-OH. Write the Lewis structure for SCl 2. Solution. Each chlorine atom has 7 valence electrons and needs one more electron to complete its valence shell. The sulfur atom has 6 valence electrons and needs two additional electrons to complete its valence shell:
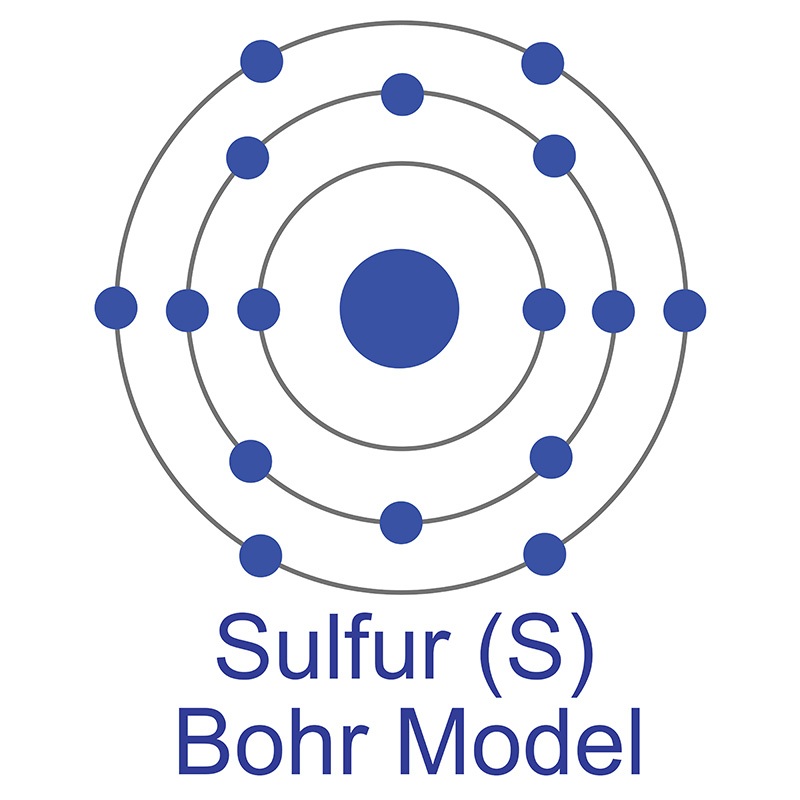
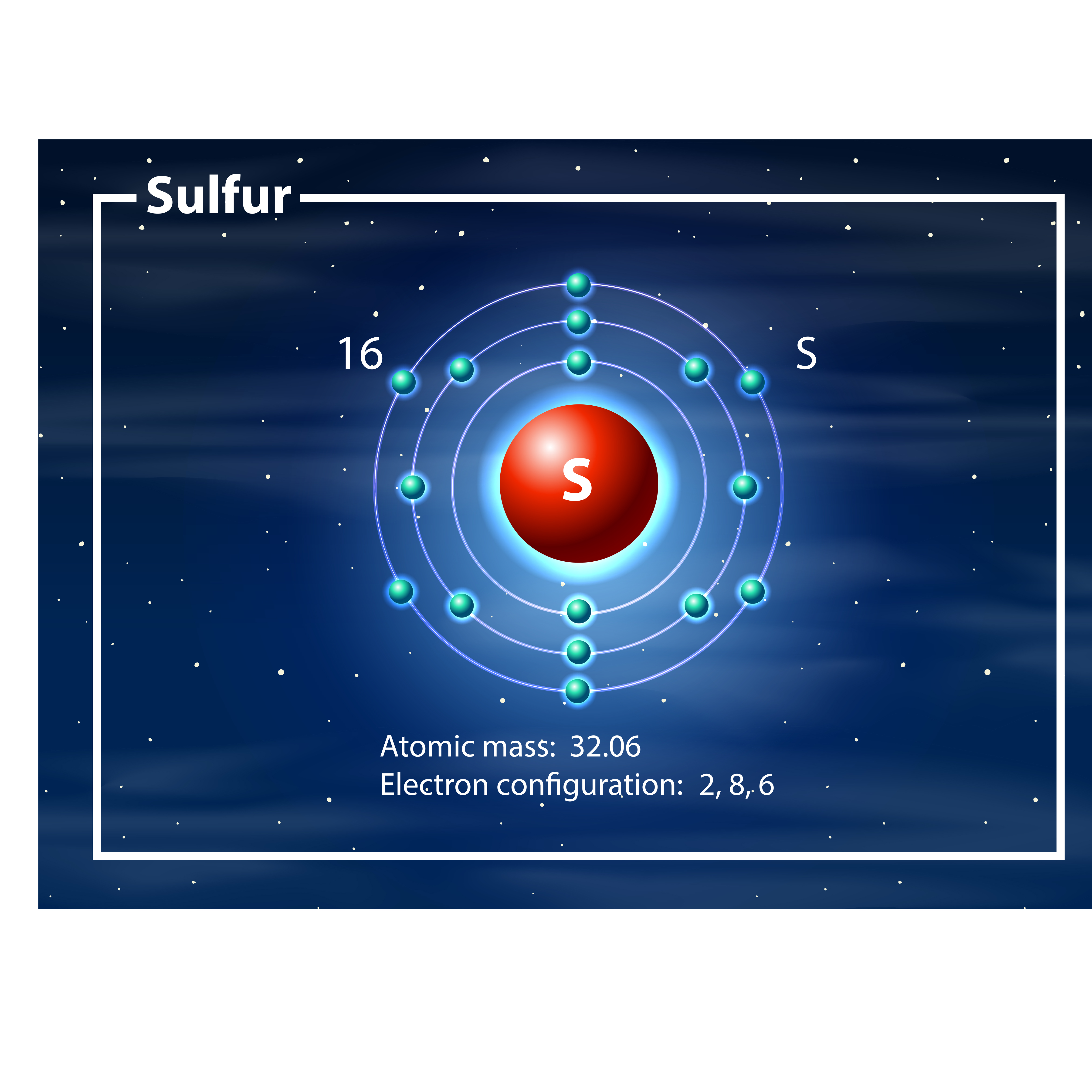
Sulfur atom diagram. Sulfur or sulphur (see spelling differences) is a chemical element with symbol S and atomic number 16. It is an abundant, multivalent non-metal. Under normal ...Electronic Shell Structure: 2, 8, 6Atomic Weight: 32.065Atomic Number: 16Electronic Configuration: 3s2 3p4Abundance · Physical Properties · Thermal Properties Lewis structure of SO3. The sulfur trioxide is a tetra atomic chemical molecule where both the sulfur and three oxygen molecules bond with an equal number of valence electrons. The diagram is drawn showing dots of valence electrons around the symbol of both sulfur and oxygen atoms with lines predicting bond formation. Structure of Sodium Metabisulfite. Sodium metabisulfite is made up of two sodium ions and one metabisulfite ion. Na+ helps to balance the charge in the structure. The metabisulfite ion is directly connected to the sulfur atoms. The first sulfur atom is bonded to three oxygen atoms and has an oxidation state of +5. In the rhombohedral allotrope, designated ρ-sulfur, the molecules are composed of rings of six sulfur atoms. This form is prepared by treating sodium ...
Lesson Summary. Sulfur trioxide is an inorganic compound that is composed of a single sulfur and three oxygen atoms. The formula for sulfur trioxide is {eq}SO_3 {/eq}. Each oxygen atom is ... With an odd number of electrons, at least one atom in the molecule will have to violate the octet rule. Examples of stable odd-electron molecules are NO, NO 2, and ClO 2. The Lewis electron dot diagram for NO is as follows: Although the O atom has an octet of electrons, the N atom has only seven electrons in its valence shell. Element Sulfur (S), Group 16, Atomic Number 16, p-block, Mass 32.06. ... ChemSpider ID, 4515054 · ChemSpider is a free chemical structure database ... The central atom is basically the atom with the highest number of bonding sites. Here, the central atom will be the sulfur atom. After finding the central atom we need to sketch a skeletal structure of H2S with single bonds only. It's good to know that lewis structure is all about electrons and atoms fulfilling their octet.
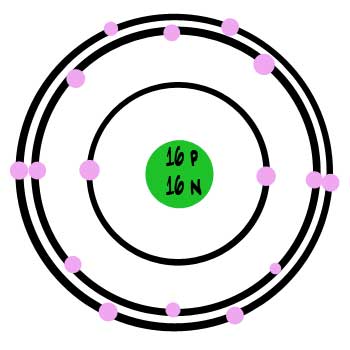
Lewis Structures: As valence electrons are significant to an atom's reactivity, it is essential to represent it by simple diagrams. Lewis structures, here, comes into the picture where the valence electrons present in an atom are represented as dots. Hence, these structures are also known as electron dot diagrams. The nucleus consists of 16 protons (red) and 16 neutrons (orange). 16 electrons (white) occupy available electron shells (rings). The stability of an element's ... Sulfur (in nontechnical British English: sulphur) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic.Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula S 8.Elemental sulfur is a bright yellow, crystalline solid at room temperature. Sulfur is the tenth most common element by mass in the universe ... 7. What specific information about the atomic structure do the periods and groups give you? 8. 5 ZnSO4 a. Count the number of Sulfur atom = b. How many total atoms are given in the compound = 9. Li2SO4 a. Count the number of Sulfur atom = b. How many total atoms are given in the compound = 10. Al2(SO3)3 a. Count the number of Sulfur atom = b.
A Lewis electron dot diagram (or electron dot diagram, Lewis dot diagram, Lewis diagram, or Lewis structure) is a representation of the valence electrons of an atom that uses dots that surround the chemical symbol of the element. The number of dots equals the number of valence electrons in the atom. Dots are arranged to the right, left, above ...
SF2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram. Sulfur Fluoride is a highly unstable inorganic compound. With a molar mass of 70.062 g/mol, this compound is made up of one Sulfur atom and two Fluoride atoms. This compound is formed when sulfur dichloride reacts at low pressure with either potassium fluoride or ...
A single atom of an element has 21 neutrons, 20 electrons, and 20 protons. Which element is it? K Zr Ca Sc Which diagram represents a gas that has bee … n ionized? diagram 1 diagram 2 diagram 3 diagram 4
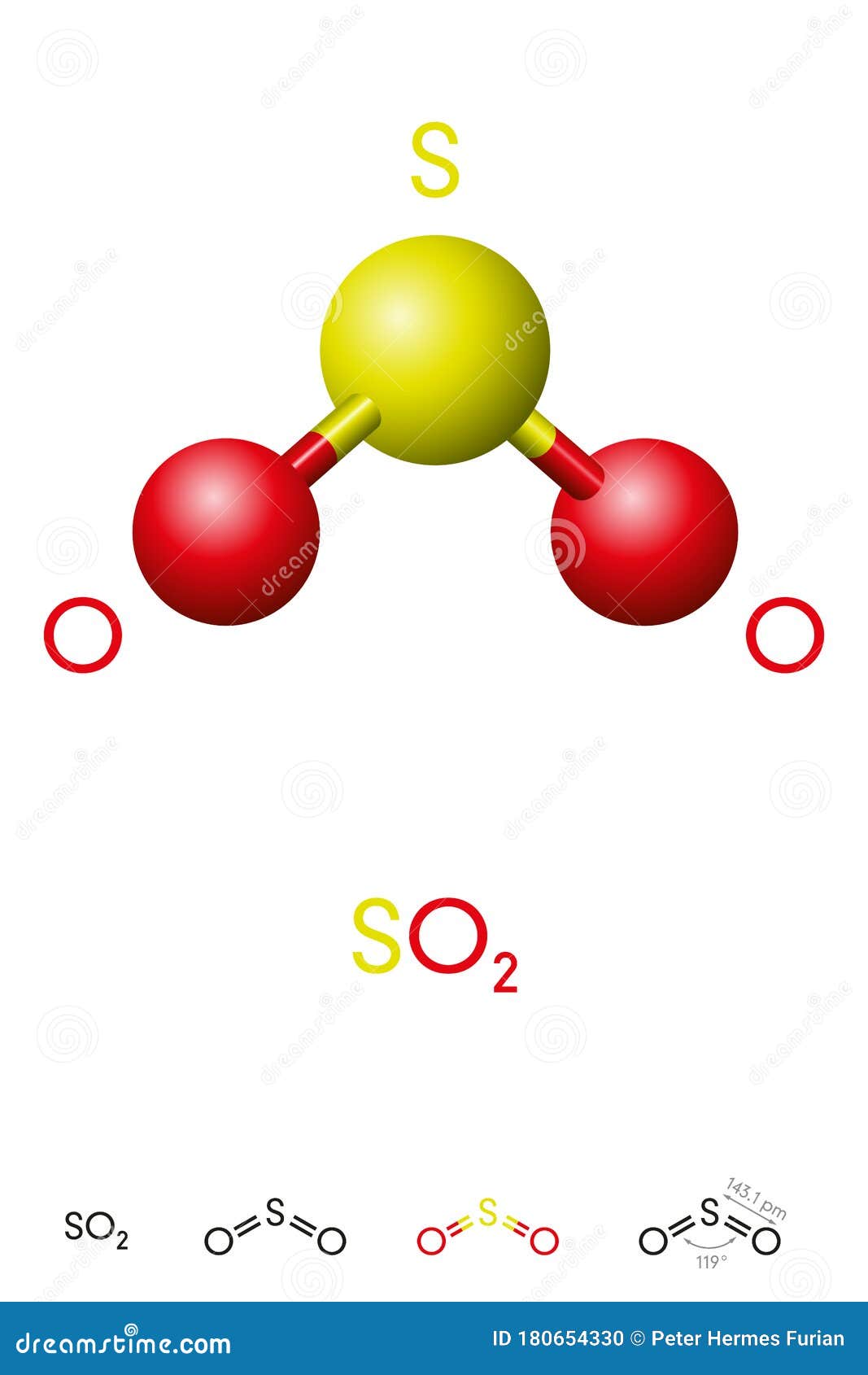
With SO2 Lewis Structure, the central atom is the central sulfur atom because of the higher valence of the sulfur atom than the oxygen atom. The SO2 Lewis Structure provides the best explanation of how the sulfuric acid (1) transformed into such after dissecting the bonds of Sulfur and Oxygen. This can be a hazard to one's health, but this is ...
Draw the Lewis structure of SCl 2 that reflects its correct three-dimensional geometry (bent or linear). Answer. SCl 2 is a bent molecule, since the central sulfur atom has two lone pairs in addition to the two covalent bonds it makes to the two chlorine atoms.
In the existing or untreated hair, the disulphide bonds join one sulfur atom in the polypeptide chain to another sulfur atom on another polypeptide chain. Reducing agents called thiol compounds add a hydrogen atom to each of these sulfur atoms in the disulfide bonds to break them. With these bonds broken, the polypeptide chains are ready to slip into a new shape.
The multianion transition metal compound is a brand-new class of substances with unique properties and high potential for energy electrocatalysis. In multianion transition metal compounds, two or more types of anions are contained and co-occupy the same type of anionic site to construct a heteroanion structure, which is visually presented as the ocean of balls in the front cover image by Li ...
The radius of a single atom of a generic element X is 199 picometers (pm) and a crystal of X has a unit cell that is face-centered cubic. Calculate the volume of the unit cell. Chemistry. Give the formal charge on the sulfur atom in a Lewis structure for the sulfate ion in which every atom satisfies the octet rule.
A Lewis dot diagram shows the valence electrons in an atom by representing them as dots arranged in pairs around the letter abbreviation for the element. Learning Outcomes Following this video ...
Apr 25, 2017 — The sulfur atom has 16 protons, 16 neutrons and 16 electrons in three different energy levels, or orbits. Physics suggests that electrons do not ...
The nitrogen atom can be bonded to either carbon or hydrogen atoms. Things look a little different, but the concept is the same for proteins; the difference is that the amide bond is formed between the amine of one amino acid and the carboxylic acid of another one (Figure 2). Figure 2. The basic structure of all amino acids that make up proteins.
Dimethyl sulfoxide is an organosulfur compound with two methyl groups, one oxygen atom and one sulfur atom. The chemical formula for dimethyl sulfoxide is (CH 3) 2 SO. It is a colourless liquid that can dissolve both polar and nonpolar compounds.Dimethyl sulfoxide is abbreviated as DMSO.
The Lewis structure of sulfurous acid has sulfur as the central atom and is shown here. This is the best Lewis structure because it does not leave formal charges on any individual atoms.
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid and semiconductor.It is a member of group 14 in the periodic table: carbon is above it; and germanium, tin, lead, and flerovium are below it. It is relatively unreactive. Because of its high chemical affinity for oxygen ...
SF6 is a colorless and odorless gas that is non-combustible and non-flammable in nature. The central atom here is sulfur bonded with 6 fluorine atoms. Lewis dot structure has 6 sigma bonds and rests lone pairs on fluorine. The hybridization of SF6 is sp3d2. SF6 has octahedral molecular geometry and is non-polar in nature.
Molecular Orbitals Formed from ns Orbitals. The molecular orbitals diagrams formatted for the dihydrogen species are similar to the diagrams to any homonuclear diatomic molecule with two identical alkali metal atoms (Li 2 and Cs 2, for example) is shown in part (a) in Figure 9.7.1 , where M represents the metal atom.Only two energy levels are important for describing the valence electron ...
The Lewis structure helps one to understand the sharing of electrons between the central and neighboring atoms within a compound. Here the central atom is Sulfur and the neighboring atoms are Fluorine. This is the general idea of how and why Lewis structures are made. SF4 Lewis Structure. Let us look at how SF4's Lewis structure can be formed.
Write the Lewis structure for SCl 2. Solution. Each chlorine atom has 7 valence electrons and needs one more electron to complete its valence shell. The sulfur atom has 6 valence electrons and needs two additional electrons to complete its valence shell:
The structure of sulfurous acid shows that the sulfur atom is bonded to two hydroxyls and a single oxygen atom. The sulfur is directly bonded with the oxygen in the hydroxyl S-OH.
6.5. Hydrogen peroxide, H2O2, reacts with sulfur trioxide to form peroxomonosulfuric acid, H2SO5, in a Lewis acid-base reaction. H2O2 + SO3 = H2SO5. c) Identify the lewis acid and base. Can someone explain how they determine the acid and base in this problem.
Design of metastable oxychalcogenide phases by topochemical (de)intercalation of sulfur in La2O2S2 | Nature Communications


















Comments
Post a Comment