42 dot diagram for iodine
Electron Dot Diagram for Iodine. which is the correct electron dot diagram for iodine plz with a pic or if u cnt wit o a pic jus tell me how it looks like electron dot diagram for iodine imageresizertool electron dot diagram for iodine moreover new website periodic table as well as watch along with 9 further also 140 explain using dot and cross in Dot and cross diagrams help us to model when ions are formed from atoms. Here's an example using sodium and chlorine. They form ions which bond to form sodium chloride. 1. Draw the electronic ...
electron dot diagram for Iodine. electron dot diagram for Boron. electron dot diagram for Sulfur. electron dot diagram for Carbon. electron dot diagram for Phosphorus. Lewis structure for PCl₃ ...

Dot diagram for iodine
iodine has 7 valence electrons. So you draw seven dots, 2 on each side of the letter I. and on one side you just put one dot, I think its best to put. The left diagram shows a Lewis dot structure of sodium with. ASK A BRAND. Likewise, they can be used .. The left shows an iodine atom with one lone pair. The orbital diagram of Iodine trichloride is given below: The Lewis structure is shown in detail in the following video. Have a look! ICl3 Polarity. For a chemical bond to be a polar covalent bond, the electronegativity difference between two atoms should be between 0.4-1.7. What is the correct Lewis dot diagram for iodine? Iodine is a halogen, and as such, it has 7 valence electrons. These will appear as dots around the "I" symbol, in three pairs and one single.
Dot diagram for iodine. What is the ionic compound formed from calcium and iodine? Calcium - metal - 2 valence electrons - loses both electrons [Ca]+2. Iodine - nonmetal - 7 valence electrons - gains 1 electron ... Topic: Lewis Dot Diagrams for Ionic Compounds Last modified by: Phosphorus And Iodine Lewis Dot Structure, P4O10 : P4o10 Lewis Dot Structure Service Unavailable In, Patente EP0547705A1 Pharmacologically active tricyclic, 6 1 Lewis Electron Dot Diagrams Introductory Chemistry, This is how the ionic bond forms in Magnesium Nitride Iodine monochloride, 99.998% trace metals basis. Q414607. Iodine monochloride solution, 1.0 M in methylene chloride. Iodine monochloride, 1M solution in dichloromethane, AcroSeal (R) Iodine monochloride, ACS reagent, 1.10+/-0.1 I/Cl ratio basis. Iodine monochloride, approx. 0.22N soln. in glacial acetic acid. What should the electron dot diagram for iodine look like? Chemistry Covalent Bonds Drawing Lewis Structures. 1 Answer Jahan Psyche Oct 24, 2015 Answer link. Related questions. What are lewis dot structures used for? What is the lewis structure for #SO_2#? How do you draw the lewis structure for ions? ...
A step-by-step explanation of how to draw the KI Lewis Dot Structure.For KI we have an ionic compound and we need to take that into account when we draw the ... Iodine dot diagram is actually amongst images libraries inside of our highest photos gallery. I wish you are going to revel in. We're going to draw the Lewis structure for I2, Iodine gas, a very pretty purple gas. So let's start out. Iodine is in group 7 of the periodic table. That means it has 7 valence electrons, so we have 7. A step-by-step explanation of how to draw the I Lewis Dot Structure.For the I structure use the periodic table to find the total number of valence electrons ... This photo about: Electron Dot Diagram for Iodine, entitled as Question Bank Electron Dot Diagram For Iodine - also describes Question Bank and labeled as: electron dot, with resolution 2455px x 1148px
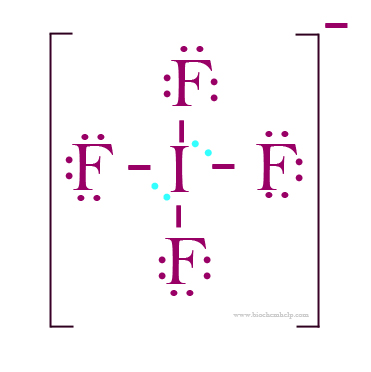
Draw a Lewis dot diagram for an iodine molecule. Draw a Lewis dot diagram for a hydrogen molecule. True or false: When drawing a covalent bond, it is proper to use a line connect atoms and a line represents 2 electrons. In your own words, describe the difference between a bond that is nonpolar covalent and polar covalent. Drawing the Lewis Structure for HI. Viewing Notes: HI is very similar to HF and HCl. Hydrogen has 1 valence electron and Iodine (in Group 7 with F and Cl) has 7 valence electrons. With the Lewis Structure for HI remember that Hydrogen only needs 2 valence electrons to have a full outer shell. Be sure that you don't use more than the 8 valence ... Precautions for safe handling: Avoid formation of dust and aerosols. Provide appropriate exhaust ventilation at places where dust is formed.Normal measures for preventive fire protection. Sigma-Aldrich; Safety Data Sheet for Iodine Monobromide. Product Number: 224847, Version 4.5 (Revision Date 08/22/2014). Since Iodine (I) is below Period 3 on the periodic table it can hold more than 8 electrons. In the Lewis structure for ICl4- the Iodine atom has 12 valence electrons. Also note that you should put the ICl4- Lewis structure in brackets with as 1- on the outside to show that it is an ion with a negative one charge.
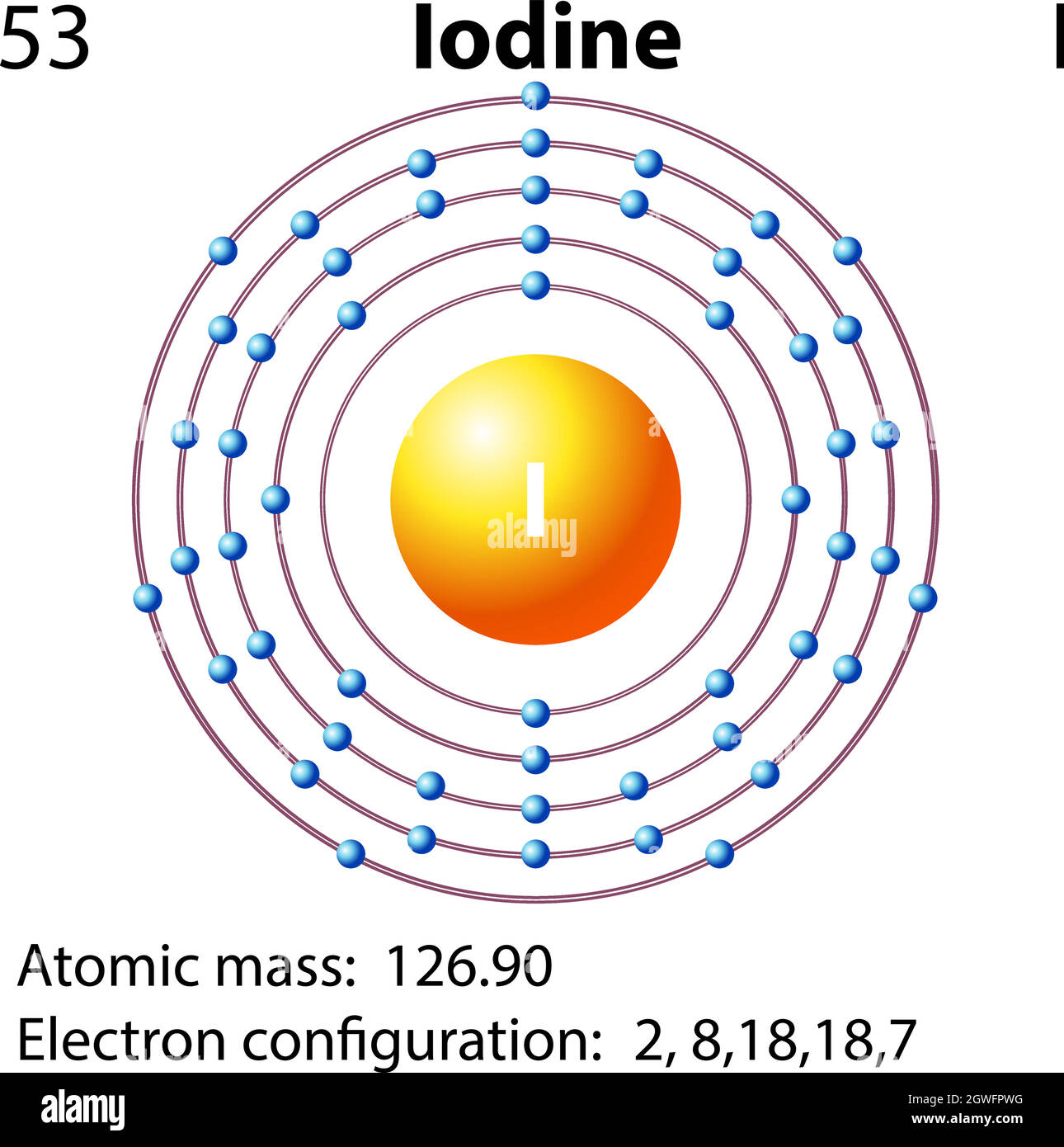
This example Total valence electrons to be "happy" = 1 iodine (8) + 3 chlorine (3 x 8). Comprehensive information for the element Iodine - I is provided by this page including scores of properties, Atomic Structure of Iodine Electron Dot Model . The left diagram shows a Lewis dot structure of sodium with. Likewise, they can be used ..

What Is The Number Of Electrons Shared Between The Atoms In I2 Molecule That Is An I Not A 1 1 Brainly Com
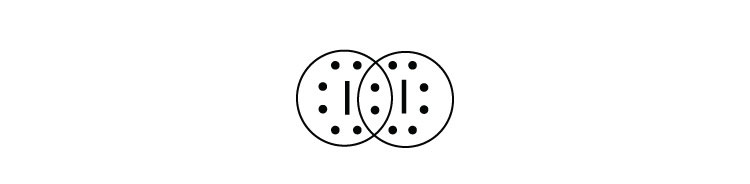
Iodine is a diatomic molecule so its molecules are paired as i2 iodine has 7 electrons in its outer shell so 1 electron is shared by each atom. To make the electron dot diagram you put the electron symbol and put a dot on one of the sides for. Chemical properties of iodine.

Lewis Dot Structures Lewis Dots Lewis Dot Structures Describe The Covalent Bonding In Molecules They Describe How The Valence Electrons Outermost S Ppt Download
Dot Structure For I2, lewis dot iodine diagram structure i2 draw, i2 electrons shared brainly pairs molecule bond atom lone between atoms each three, molecular shape geometry chem bonded lewis i2 atom central octet tutor scientific atoms examples, molecular geometry shape i2 n2 octet atoms atom chem lewis tutor bonded scientific central
CHEMISTRY LAB: ELECTRON DOT DIAGRAMS FOR IONIC COMPOUNDS WHAT TO TURN IN: Data Table Questions #1-5 Objectives • To review element and ion names and symbols ... magnesium and iodine MgI 2 3) aluminum and fluorine AlF 3 4) calcium and nitrogen Ca 3N2 5) zinc and selenium ZnSe (Note: zinc is a typical transition metal.) ...
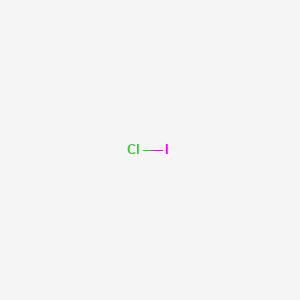
For Iodine we have 7 valence electrons, and 7 for the Chlorine; total of 14 valence electrons for the ICl Lewis structure. We'll put the Iodine here, and the Chlorine right next to it. We have a total of 14 valence electrons. We'll put 2 between atoms to form the chemical bond, and we'll go around the outside.

Symbol And Electron Diagram For Iodine Illustration Royalty Free Cliparts Vectors And Stock Illustration Image 46349295
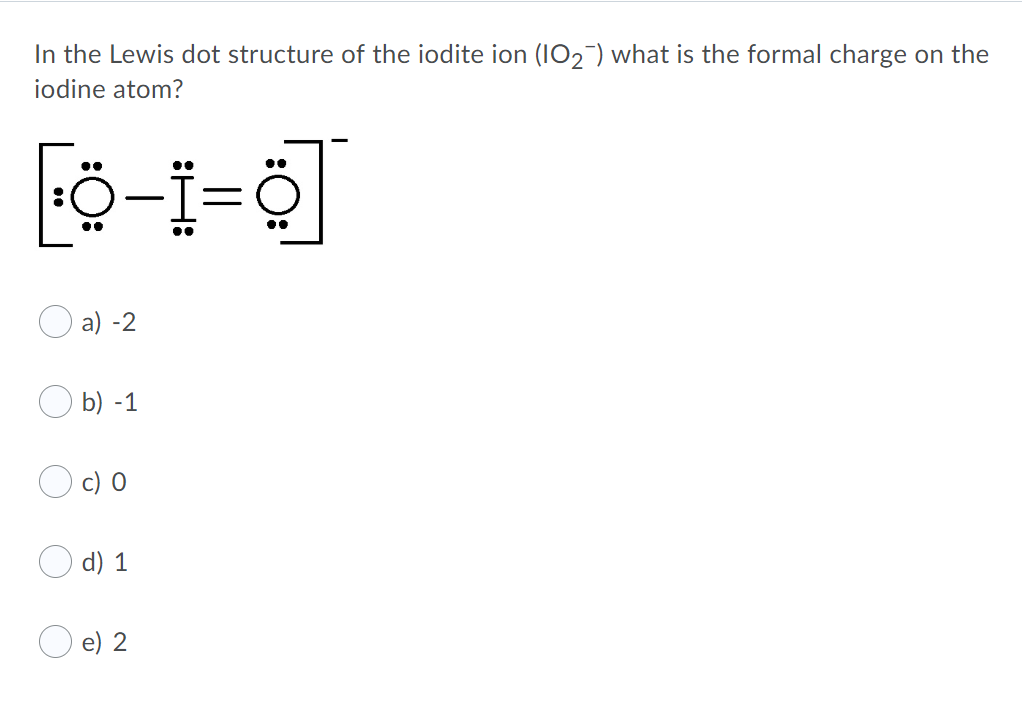
Iodine Dot Diagram What Is The Formal Charge On The Iodine Atom In Icl2 Where Iodine Is The Central Atom A 2 B 1 C 0 D 1 E 2. Iodine Dot Diagram Name Hour Draw A Dot Diagram For The Following Elements 1. Iodine Dot Diagram Periodic Table Ions New Lewis Dot Diagram For Iodine Luxury Lewis.
When Magnesium and Iodine are combined with some water, a violet vapor is produced. The reaction of Magnesium metal and Iodine will 'yield' Magnesium Iodide. The two elements will create an ionic bond (MgI2). The two valence electrons of Mg will be distributed to each of the Iodine atoms.
Hint: The electron dot diagram is the diagram which clearly shows the bonding between the atoms of a molecule along with the lone pairs of electrons, if they exist in the molecule. Iodine is the molecule consisting of two atoms of iodine bonded together satisfying both the planes. Complete answer: Let us study the concept;
Draw a Lewis dot diagram for an iodine molecule. 2. Draw a Lewis dot diagram for a hydrogen molecule. 3. True or false: When drawing a covalent bond, it is proper to use a line connect atoms and a line represents 2 electrons. 4. In your own words, describe the difference between a bond that is nonpolar covalent and polar covalent. Cite one ...
The left diagram shows a Lewis dot structure of sodium with. Likewise, they can be used .. The left shows an iodine atom with one lone pair. When we write the . Comprehensive information for the element Iodine - I is provided by this page including scores of properties, Atomic Structure of Iodine Electron Dot Model .

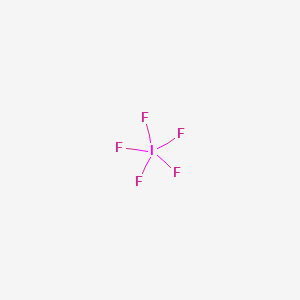
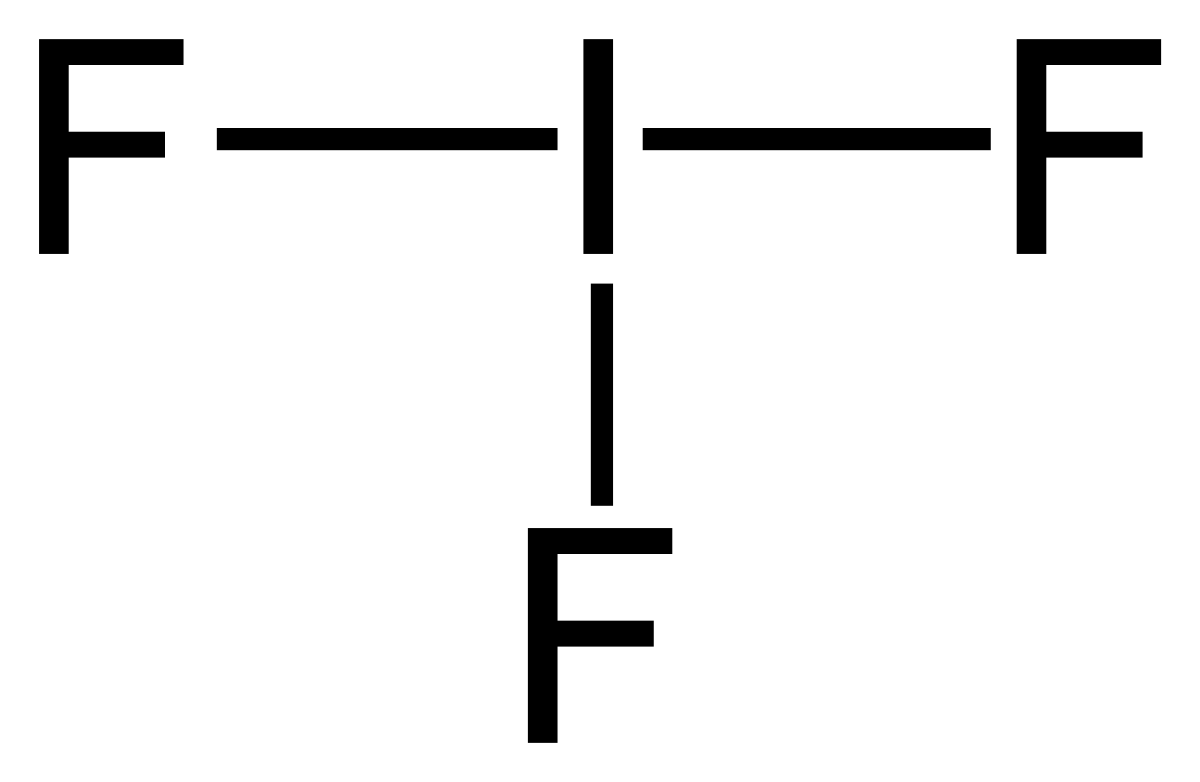
Lewis Structure Bromine Pentafluoride Sulfur Tetrafluoride Xenon Tetrafluoride Iodine Pentafluoride Citing Angle Text Number Png Pngwing
The lewis dot diagram for platinum is a diagram showing bonds electrons of the platinum atom within a molecule. That means it has 7 valence electrons so we have 7. Iodine is in group 7 of the periodic table. Iodine is in group 17 on the periodic table meaning that iodine needs only a single electron to become chemically stable.
What is the correct Lewis dot diagram for iodine? Iodine is a halogen, and as such, it has 7 valence electrons. These will appear as dots around the "I" symbol, in three pairs and one single.
The orbital diagram of Iodine trichloride is given below: The Lewis structure is shown in detail in the following video. Have a look! ICl3 Polarity. For a chemical bond to be a polar covalent bond, the electronegativity difference between two atoms should be between 0.4-1.7.
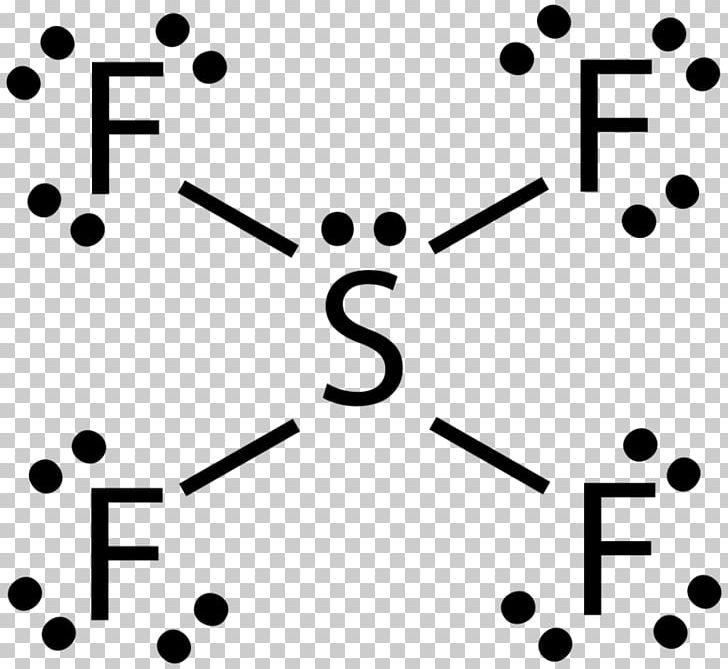
Lewis Structure Bromine Pentafluoride Sulfur Tetrafluoride Xenon Tetrafluoride Iodine Pentafluoride Png Clipart Angle Area Black And
iodine has 7 valence electrons. So you draw seven dots, 2 on each side of the letter I. and on one side you just put one dot, I think its best to put. The left diagram shows a Lewis dot structure of sodium with. ASK A BRAND. Likewise, they can be used .. The left shows an iodine atom with one lone pair.

I Iodine Element Information Facts Properties Trends Uses And Comparison Periodic Table Of The Elements Schoolmykids

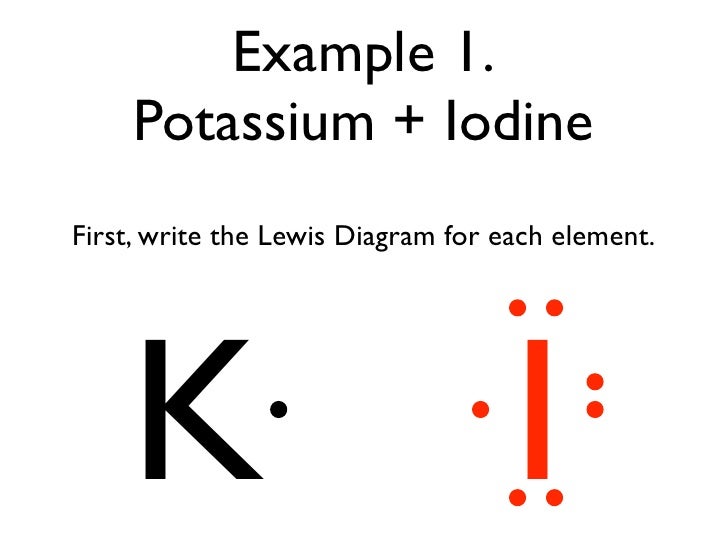
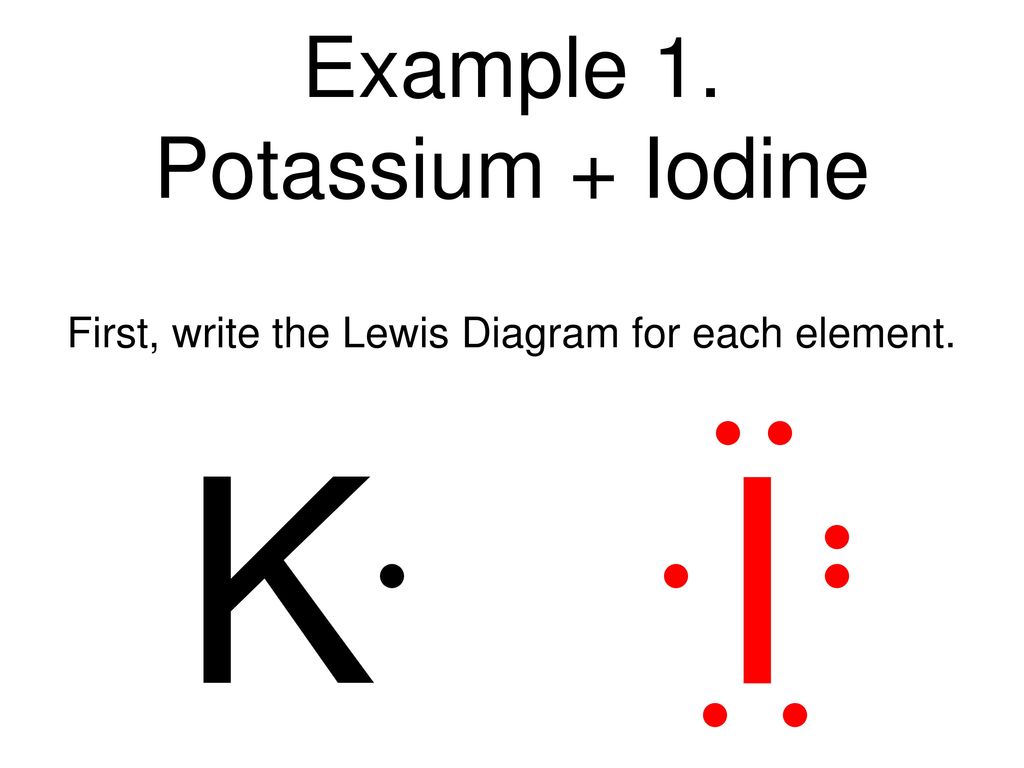
12 Use Electron Dot Structures To Determine Formulas Of The Ionic Compounds Formed When A Potassium Reacts With Iodine K I K 1 I B Course Hero




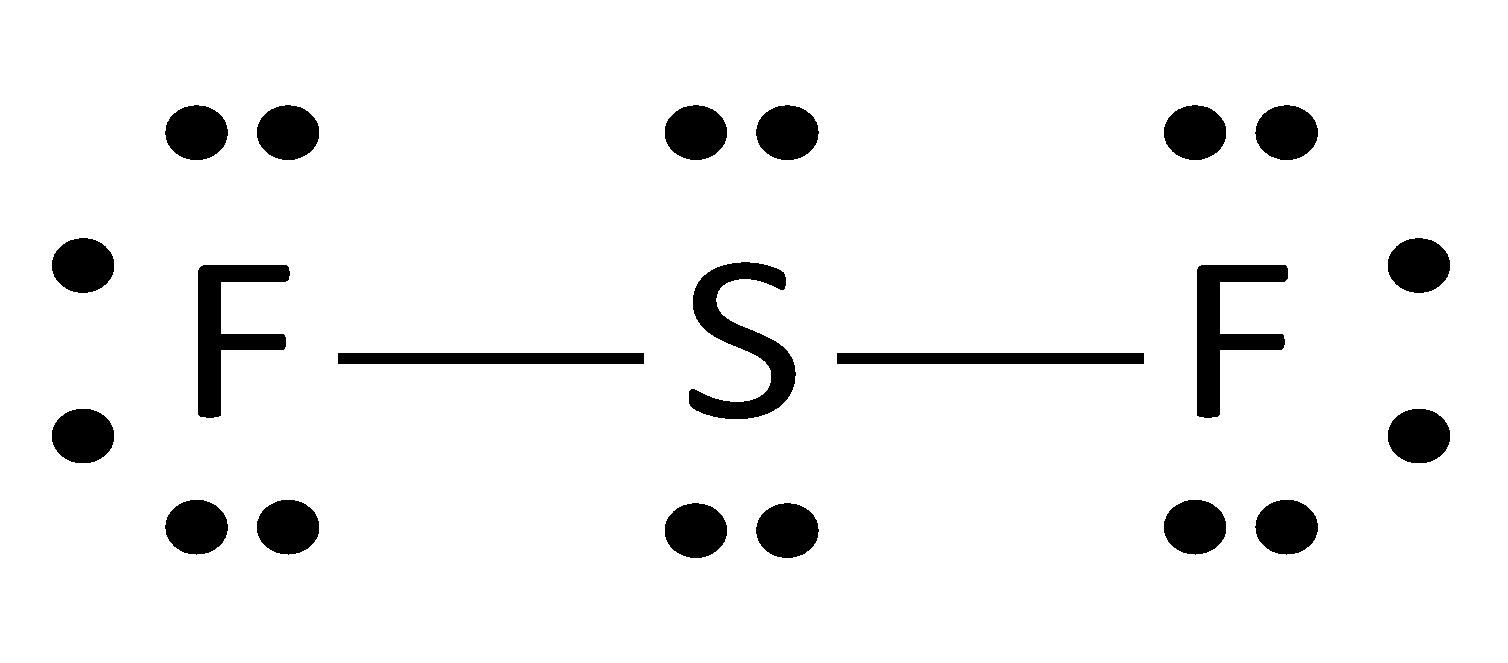







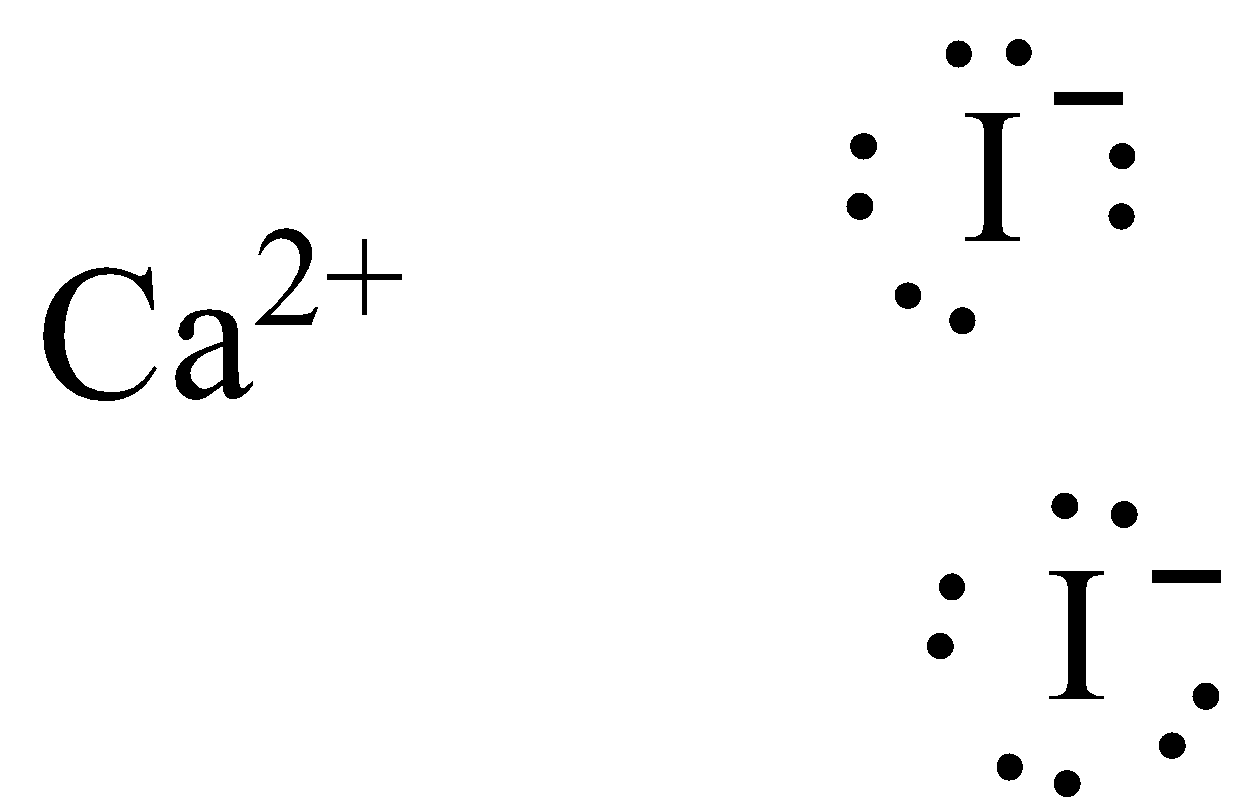

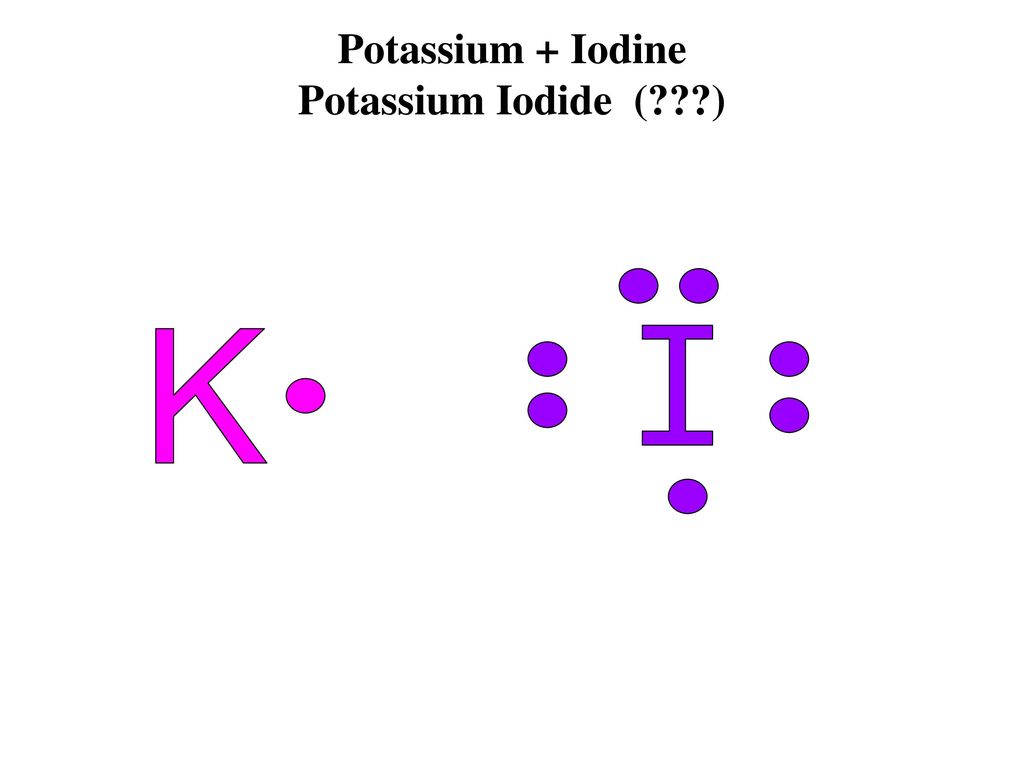
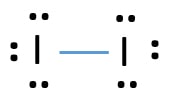



Comments
Post a Comment