42 reaction energy diagram organic chemistry
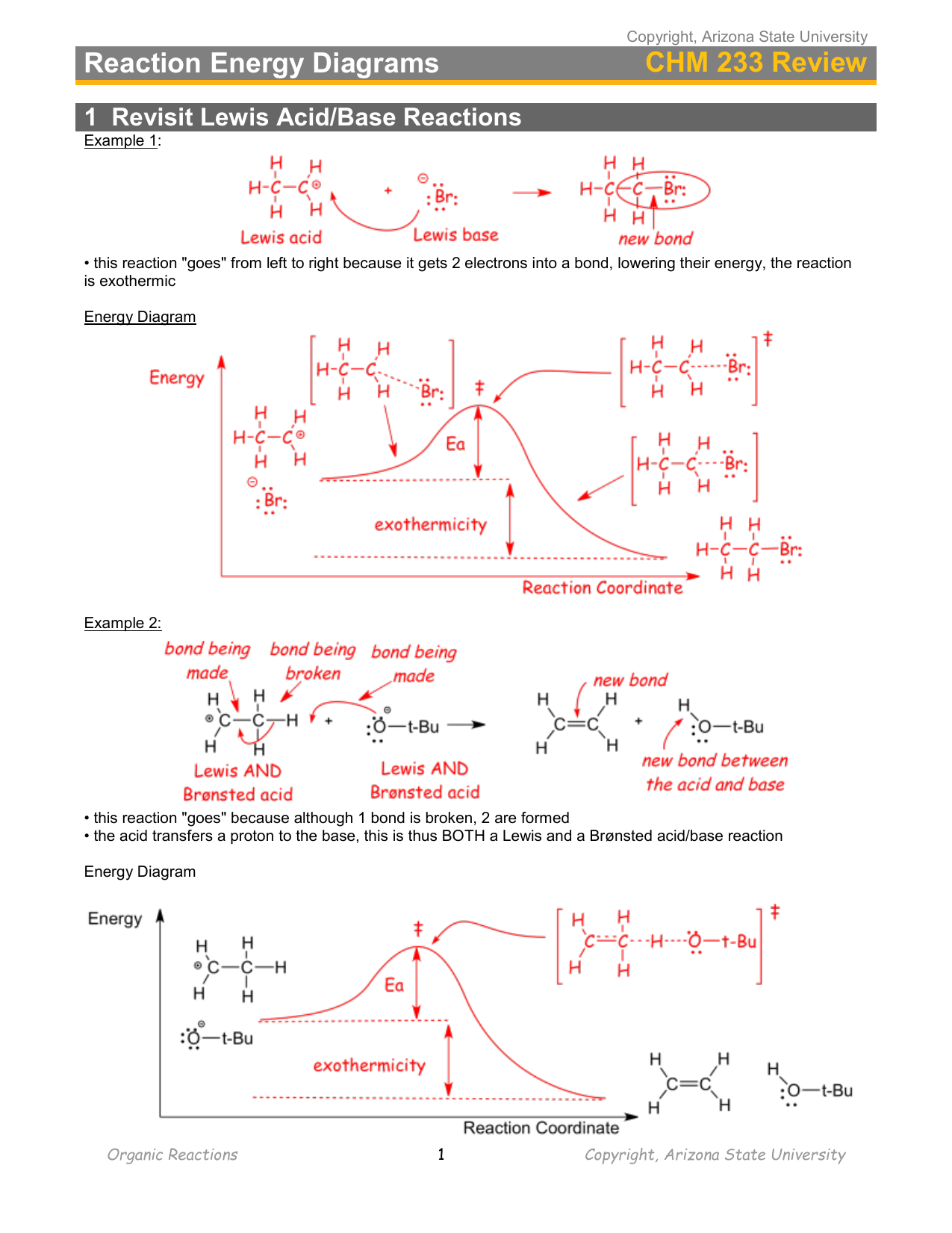
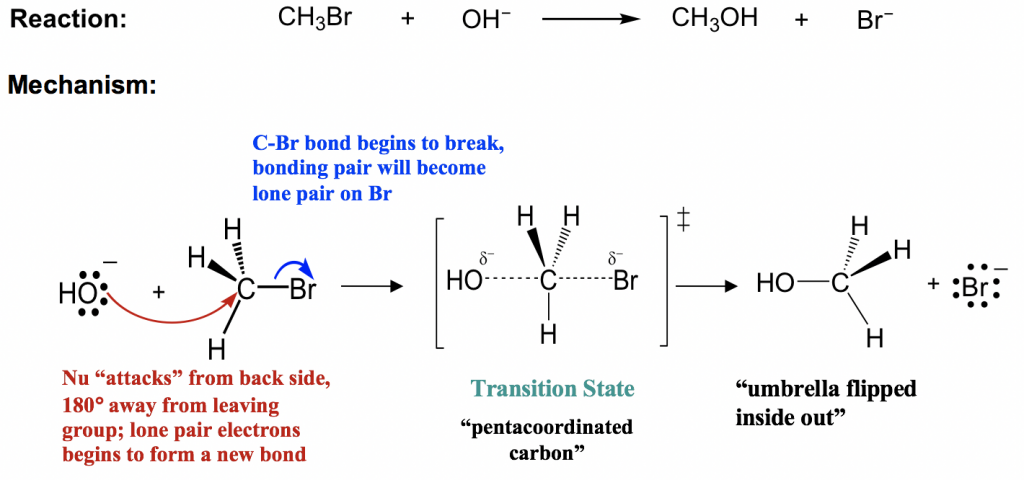
Chemistry 5.12 Spring 2003, Handout #9 Substitution Reactions (S N2 versus S N1) ... reaction coordinate (E1) energy ... B - X - H R R R R B CHR 2 X R R δ− δ+ Bimolecular Unimolecular Substitution Elimination Generic Reaction-Energy Diagrams Predicting the Products: Substitution versus Elimination Is Nuc/Base strong? no Unimolecular ... that must break during a reaction. An electron sink is an atom on a molecule or ion that can accept a new bond or lone pair of electrons. Learning to identify the characteristic sources and sinks in different functional groups is the key to learning organic chemistry reaction mechanisms. For example, for
Potential energy diagrams are images that relay the energy changes as a reaction progresses from start to finish. ... Organic Chemistry Physics ... Hence highter is the activation energy, lower is the rate of the reaction

Reaction energy diagram organic chemistry
This video is part of a 27-lecture undergraduate-level course titled "Organic Chemistry" taught at UC Irvine by Professor James Nowick. Recorded November 13, 2009. Index of Topics:-1:32 Single Step Substitution Reaction Example-3:26 Multi-step Substitution Reaction Example-11:11 Energy Diagram for Single Step Reaction E1 Reaction Mechanism and E1 Practice Problems. The E1 is a stepwise, unimolecular - 1st order elimination mechanism: The first, and the rate-determining step is the loss of the leaving group forming a carbocation which is then attacked by the base: This is similar to the S N 1 mechanism and differs only in that instead of a nucleophilic ... 4. These reactions are initiated either by heat or light. Such class of reactions are called as pericyclic reactions. Reactions are either carried out thermally or photochemically. The chemical reaction in which starting material is converted into a single stereo-isomeric product is called as stereospecific or regiospecific reaction. Factors ...
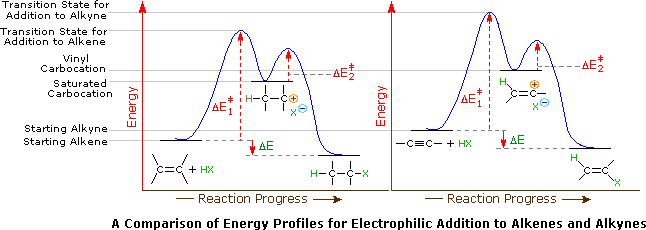
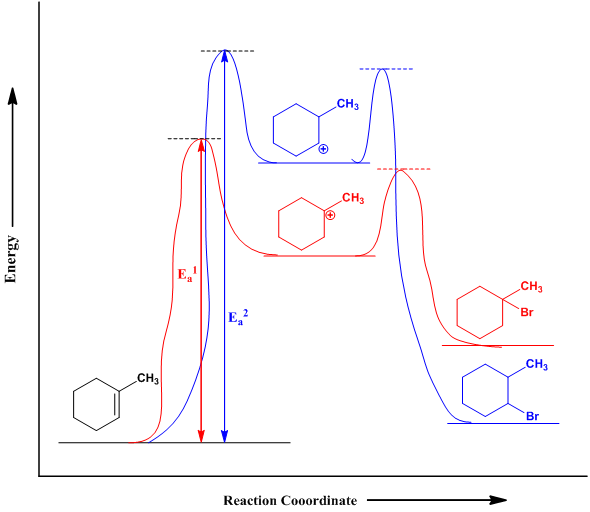
Reaction energy diagram organic chemistry. 9.4 Chlorination vs Bromination 9.4.1 Monochlorination. First we will focus on monochlorination product, by assuming that chlorination only occur once. Since chlorine is a rather reactive reagent, it shows relative low selectivity, that means Cl 2 does not discriminate greatly among the different types of hydrogens atoms (primary, secondary or tertiary) in an alkane. Part I Library of Synthetic Reactions 1 Note that this is a partial list of reactions 1 Graphics are obtained mostly from Stony Brook University CHE 327 PowerPoint slides and Organic Chemistry , 10th Edition by Solomons and ryhle.F 3 Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams. Reactions of Alkenes & Alkynes Addition Reactions of Alkenes. The most common chemical transformation of a carbon-carbon double bond is the addition reaction.A large number of reagents, both inorganic and organic, have been found to add to this functional group, and in this section we shall review many of these reactions.
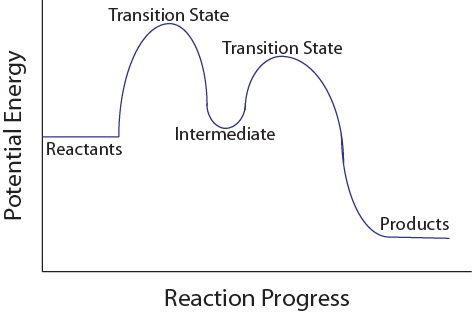
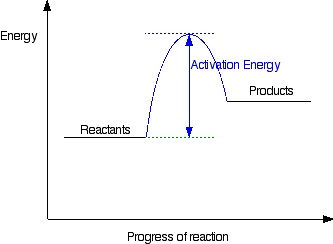
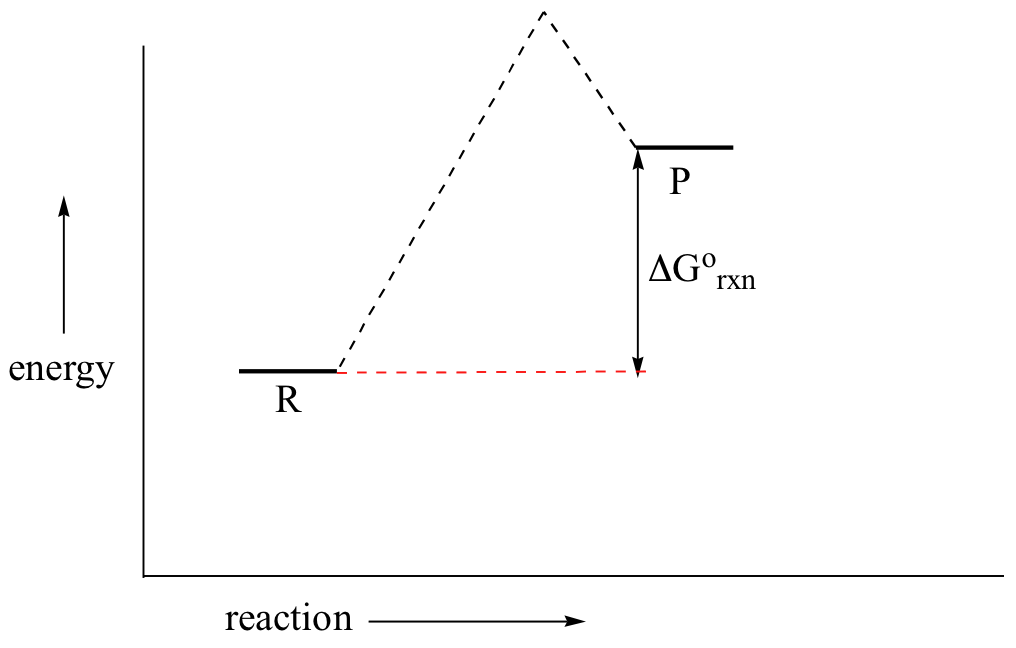
6.7: Energy Diagrams. You may recall from general chemistry that it is often convenient to describe chemical reactions with energy diagrams. In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ' reaction coordinate ', tracing from left to right the progress of the reaction ... Under this assumption, the activation energy barrier for A (higher energy conformation) should be less than that for B, which should explain the observed reaction rates. Having said that, I would not necessarily think that energy diagrams are the best way to explain this difference in reactivity, or indeed if there is a single, simple explanation. The kinetics has to do with the activation energy that it takes in order to make a reaction go forward. The activation energy in the free-energy diagram I gave you above would be the difference in the energy between the beginning and your highest point in the reaction. This would be my activation energy. Energy changes in a reaction can be illustrated by Energy Diagrams in which the highest energy point in a reaction step is called the transition state and the energy required to go from reactant to transition state is the activation energy. If a reaction occurs in more than one step, it must involve species that are neither the reactant nor the ...
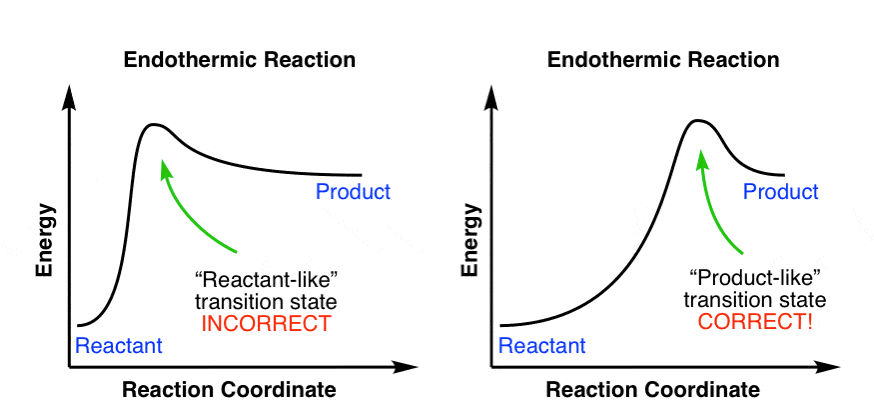
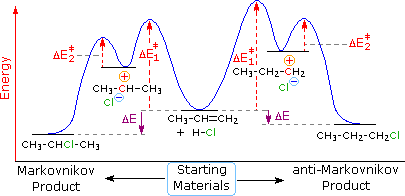
This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f... in the energy diagram)! Selectivity: if more than one site is available for reaction the ratio between each product obtained determines the selectivity! (the difference in E a for each competing path in the energy diagram)! This leads to an almost universal statement in organic chemistry:! FOR A GIVEN REACTION THE MORE REACTIVE, THE LESS SELECTIVE! Energy changes accompany chemical reactions. Energy diagrams are quite useful in illustrating these changes on a continuous basis as the reaction proceeds. Terms such as "activation energy" (E a), "transition state" (*), and "enthalpy change" are easy to define by referring to a graph such as Figure 1. Endothermic and exothermic reactions are ... Energy Level Diagram of an Endothermic Reaction. The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react.
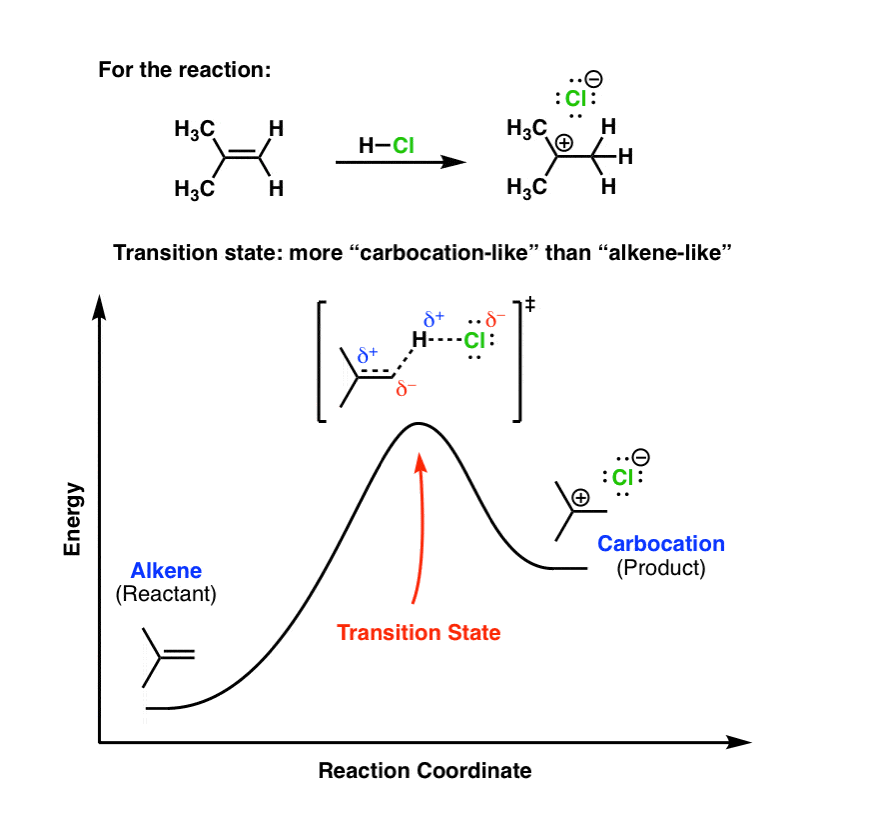
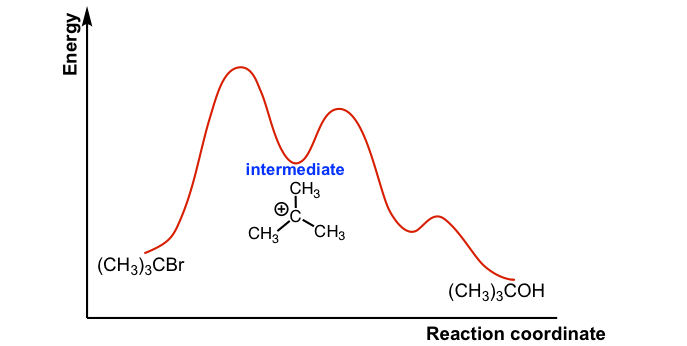
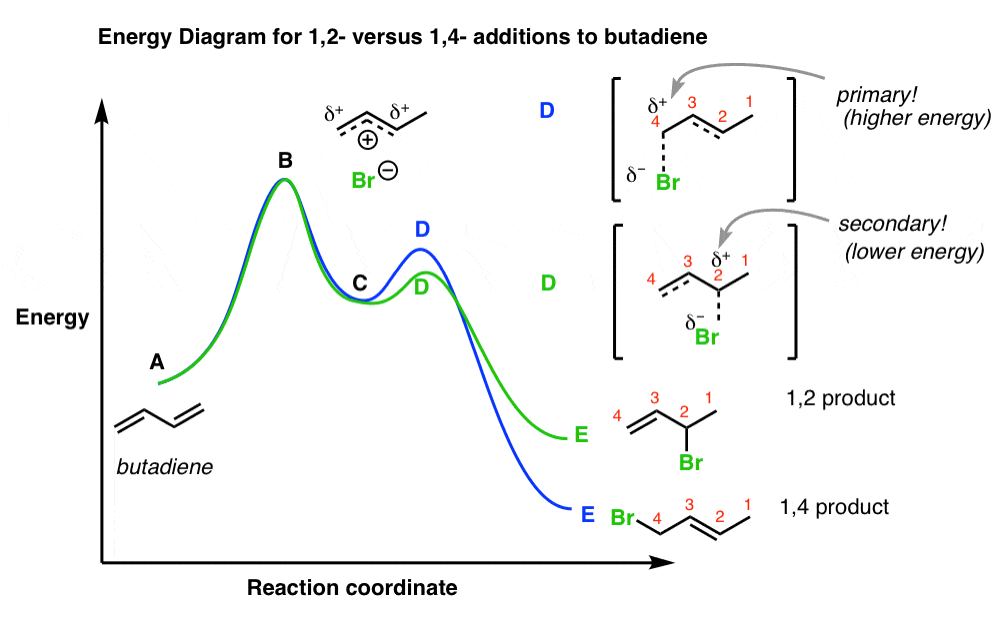
Type 2 is to count the number of intermediates, transitions states, or the mechanistic steps in the reaction based on the diagram. The reaction diagram above has 2 intermediates and 3 transition states, so it is a 3-step reaction. Finally, the last question you can expect is a question about the shape or a nature of the transition state itself.
This video looks at the features of a reaction energy diagram and how you can use it to interpret and understand the mechanism of a reaction.
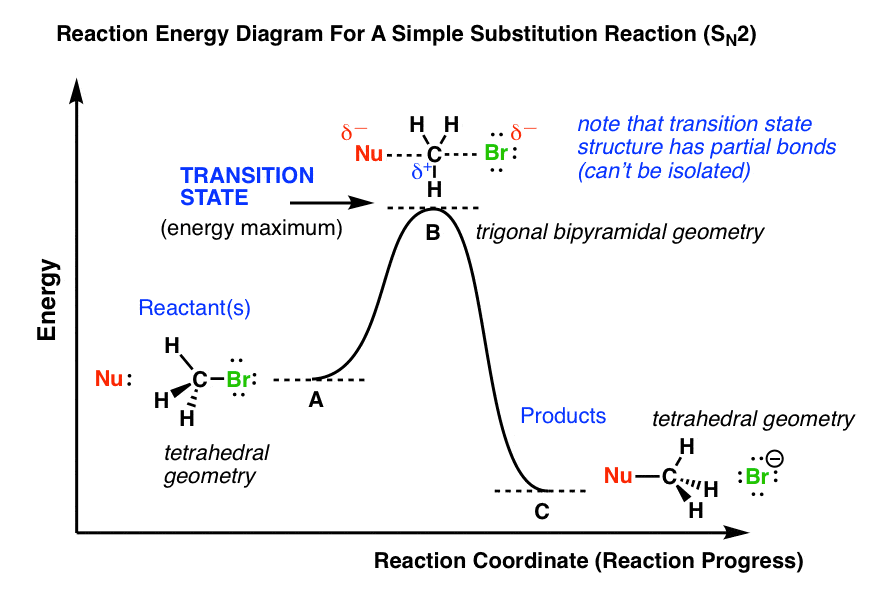
3) (12 Pts) Please draw an energy diagram for a thermodynamically favorable Sn2 reaction. Clearly indicate the starting material, transition state(s) (+), intermediates(s), product, AG and EA of the reaction (show where they can be determined from the diagram). Then provide a transition state structure in the provided brackets.
Study Guide with Solutions Manual for McMurry's Fundamentals of Organic Chemistry (7th Edition) Edit edition Solutions for Chapter 3 Problem 18P: Sketch reaction energy diagrams to represent each of the following situations. (a) A reaction that releases energy and takes place in one step (b) A reaction that absorbs energy and takes place in one ...
energy diagram (or reaction energy diagram): a graph of the energy of a reaction against the progress of the reaction. enthalpy: a thermodynamic state function, generally measured in kilojoules per mole. In chemical reactions the enthalpy change (deltaH) is related to changes in the free energy (deltaG) and entropy (deltaS) by the equation:
Organic Chemistry Symbols. The vector stencils library "Chemical elements" contains 118 icon symbols of chemical elements. Use these shapes for drawing atoms, structural formulas of inorganic and organic molecules and ions, and schemes of chemical reaction mechanisms in the ConceptDraw PRO diagramming and vector drawing software extended with ...
Reaction Energy Diagrams (17 min) This tutorial introduces the concept of Reaction Energy Diagrams and the wealth of information that they provide. (Runtime = 16:47) • Identify the various parts of a reaction energy diagram and determine whether the reaction, as well as each steps of the reaction, is endothermic or exothermic.
You may recall from general chemistry that it is often convenient to describe chemical reactions with energy diagrams. In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the 'reaction coordinate', tracing from left to right the progress of the reaction from starting compounds to final products.

Is This Really A Comparison Of The Absolute Energies In The Reaction Energy Profile For Ester Formation Chemistry Stack Exchange
Reaction Energy DiagramsReaction Energy Diagrams If a reaction occurs in more thiilhan one step, it must involve species that are neither the reactant nor the final product Th ll dThese are called reaction intermediates or simply "intermediates" Eh h i fEach step has its own free energy of activation The complete diagram for the
• Grignard reaction followed by oxidation essentially substitutes a carbon group for a hydrogen 1 1 OH.PC 2. PhMgBr ... General Recognition of Oxidation/Reduction in Organic Chemistry CO R H R 2º alcohol RH O Aldehyde OH Carboxylic Acid RR O Ketone or H CO H H R 1º alcohol H or oxidation reduction ... 6 CO2 + 6 H2O + energy s ugars C6H6(OH ...

How Can I Draw A Simple Energy Profile For An Exothermic Reaction In Which 100 Kj Mol 1 Is Evolved And Which Has An Activation Energy Of 50 Kjmol 1 Socratic
For a chemical reaction or process an energy profile (or reaction coordinate diagram) is a theoretical representation of a single energetic pathway, along the reaction coordinate, as the reactants are transformed into products. Reaction coordinate diagrams are derived from the corresponding potential energy surface (PES), which are used in computational chemistry to model chemical reactions by ...
4. These reactions are initiated either by heat or light. Such class of reactions are called as pericyclic reactions. Reactions are either carried out thermally or photochemically. The chemical reaction in which starting material is converted into a single stereo-isomeric product is called as stereospecific or regiospecific reaction. Factors ...
E1 Reaction Mechanism and E1 Practice Problems. The E1 is a stepwise, unimolecular - 1st order elimination mechanism: The first, and the rate-determining step is the loss of the leaving group forming a carbocation which is then attacked by the base: This is similar to the S N 1 mechanism and differs only in that instead of a nucleophilic ...
This video is part of a 27-lecture undergraduate-level course titled "Organic Chemistry" taught at UC Irvine by Professor James Nowick. Recorded November 13, 2009. Index of Topics:-1:32 Single Step Substitution Reaction Example-3:26 Multi-step Substitution Reaction Example-11:11 Energy Diagram for Single Step Reaction



.png)











Comments
Post a Comment