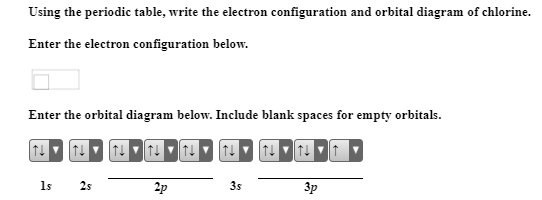
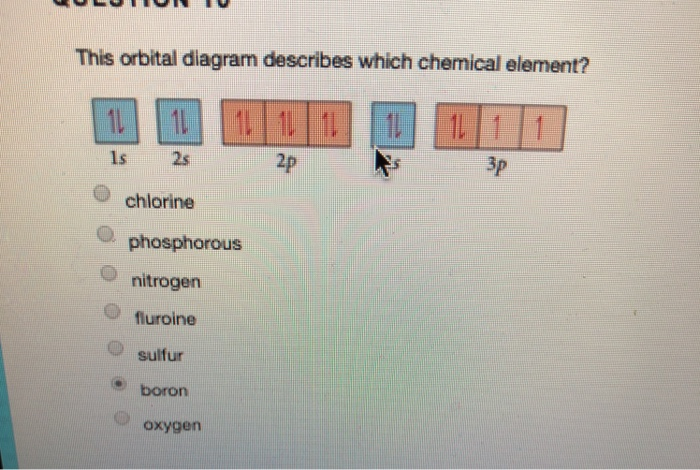
43 orbital diagram for chlorine
So I need someone to check some of these, so I might crosspost this to other subreddits, if you know any, please do. Or if you are an expert yourself, please correct me if there's any mistakes. But I did watch Dr. Stone in an *Anime Streaming website*, I posted some interesting comments in the discussions of Dr. Stone Episodes. I will post them in a chronological order with the matching episodes. Although, I think it's a bad idea to post this in a whole one post. Because no one gonna read it t... Chlorine (Cl) has an atomic mass of 17. Find out about its chemical and ... Electron Configuration, [Ne] 3s2 3p5. 1s2 2s2 2p6 3s2 3p5. Orbital Diagram.
I have a homework problem asking me to construct the molecular orbital diagram for methylene chloride, and I am not too sure what to do next. I have determined that all of the orbitals transform as follow: C 2S=A1 C 2Pz=A1 C 2Py=B2 C 2Px=B1 2H 1S=A1+B2 2Cl 3S=A1+B1 2Cl 3Pz=A1+B1 2Cl 3Py=A2+B2 2Cl 3Px=A1+B1 My thoughts were to construct the MO for the CH2 side, then add the two chlorines from there. Let me know if you have any pointers. thank you

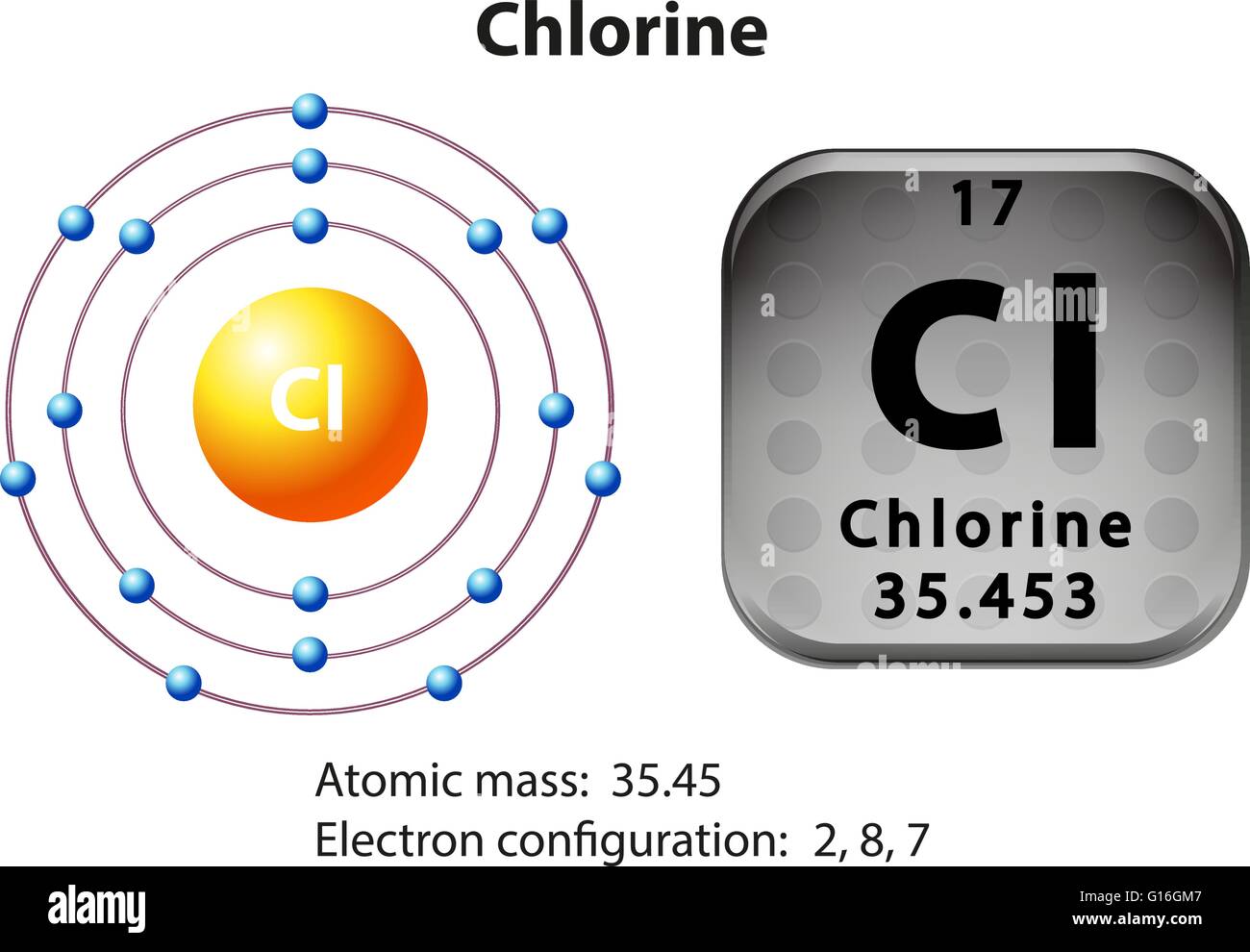
Orbital diagram for chlorine
Hey All, I'm new to the forum here and a struggling O-Chem student. I'm taking OChem 1, and although I do enjoy it, I'm still timid about my answers. I'm doing a test review problems, and I was wondering if I could get some help to verify my answers. I'm not hundred percent sure, and any inputs would be greatly appreciated. Anything that isn't clear, too vague, etc. Thank you in advance! :) (**4**). Explain why free radical halogenation produces racemic mixtures of products. -Because the ra... Hey All, I'm new to the forum here and a struggling O-Chem student. I'm taking OChem 1, and although I do enjoy it, I'm still timid about my answers. I'm doing a test review problems, and I was wondering if I could get some help to verify my answers. I'm not hundred percent sure, and any inputs would be greatly appreciated. Anything that isn't clear, too vague, etc. Thank you in advance! :) (**4**). Explain why free radical halogenation produces racemic mixtures of products. -Because the ra... Hey, guys, Welcome back. All right, so in this practice problem, we are asked to draw a new atomic orbital diagram for chlorine.20 Feb 20181 answer · Top answer: Chlorine• Atomic # 17 : 17 electrons• Electron Configuration: 1s22s22p63s23p5 [readmore]Atomic Orbital Diagram• Lowest energy orbital at the bottom• ...
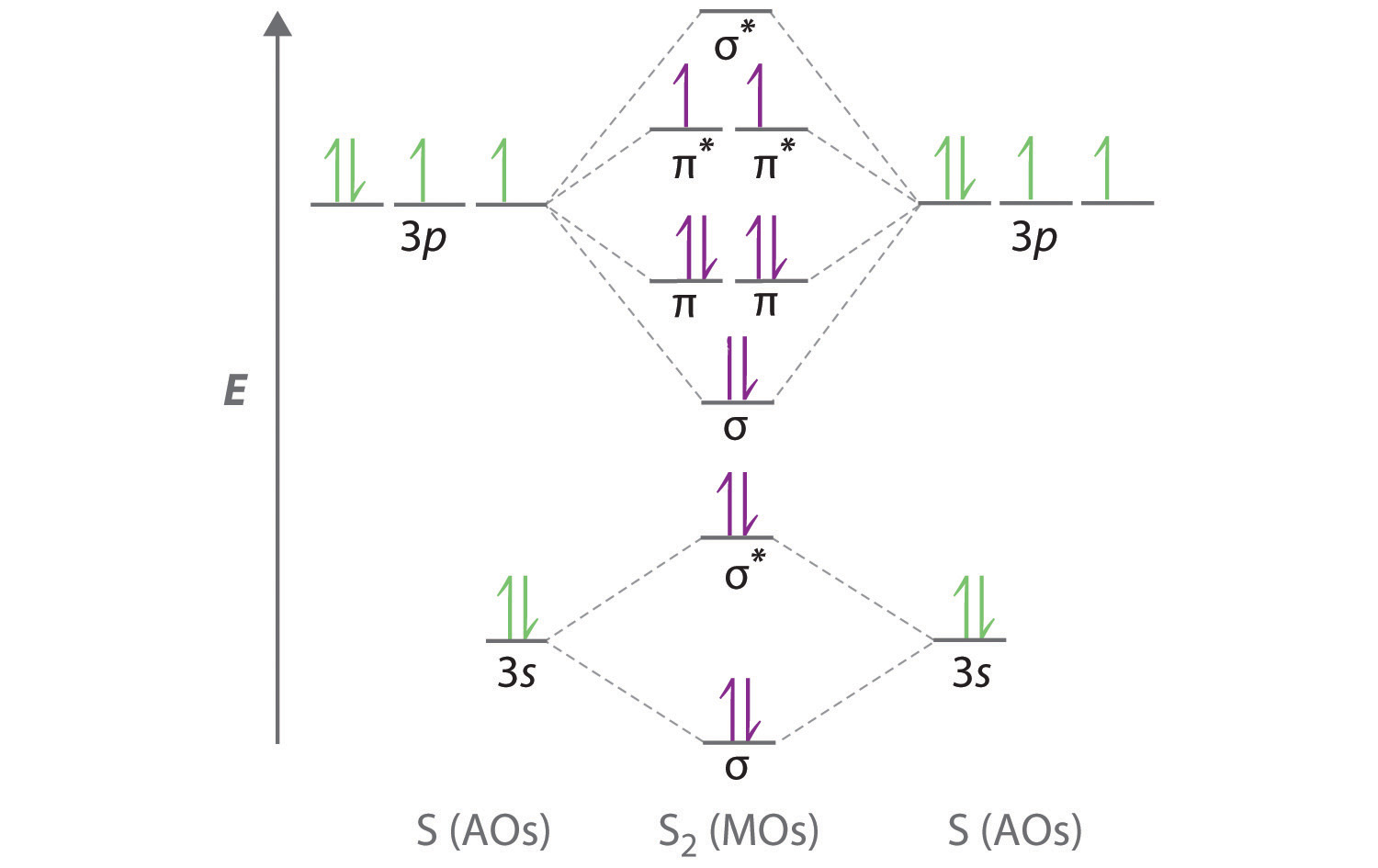
Orbital diagram for chlorine. Hey All, I'm new to the forum here and a struggling O-Chem student. I'm taking OChem 1, and although I do enjoy it, I'm still timid about my answers. I'm doing a test review problems, and I was wondering if I could get some help to verify my answers. I'm not hundred percent sure, and any inputs would be greatly appreciated. Anything that isn't clear, too vague, etc. Thank you in advance! :) (**4**). Explain why free radical halogenation produces racemic mixtures of products. -Because the ra... Here, we have to make an orbital diagram of chloride ions. Chlorine atom belongs to halogen family, the electronic configuration of halogen family a is ...1 answer · Top answer: Hint: In a neutral atom, numbers of electron are always equal to number of proton because electron contains negative charge while proton contains ... Draw a fully labelled molecular orbital energy level diagram for the molecule SCl. Show only the molecular orbitals made from the sulfur 3p orbitals and the chlorine 3p orbitals (you can use boxes or lines to indicate orbital energy levels). Label the molecular orbitals as σ or π and indicate whether they are bonding or antibonding orbitals. You can assume that the 3p S atomic orbitals are of slightly higher energy than the 3p Cl atomic orbitals in your diagram. i.Calculate the bond order for S... Nov 01, 2021 · Orbital diagram of Chlorine (Cl) 18: Orbital diagram of Argon (Ar) 19: Orbital diagram of Potassium (K) 20: Orbital diagram of Calcium (Ca) 21: Orbital diagram of Scandium (Sc) 22: Orbital diagram of Titanium (Ti) 23: Orbital diagram of Vanadium (V) 24: Orbital diagram of Chromium (Cr) 25: Orbital diagram of Manganese (Mn) 26: Orbital diagram ...
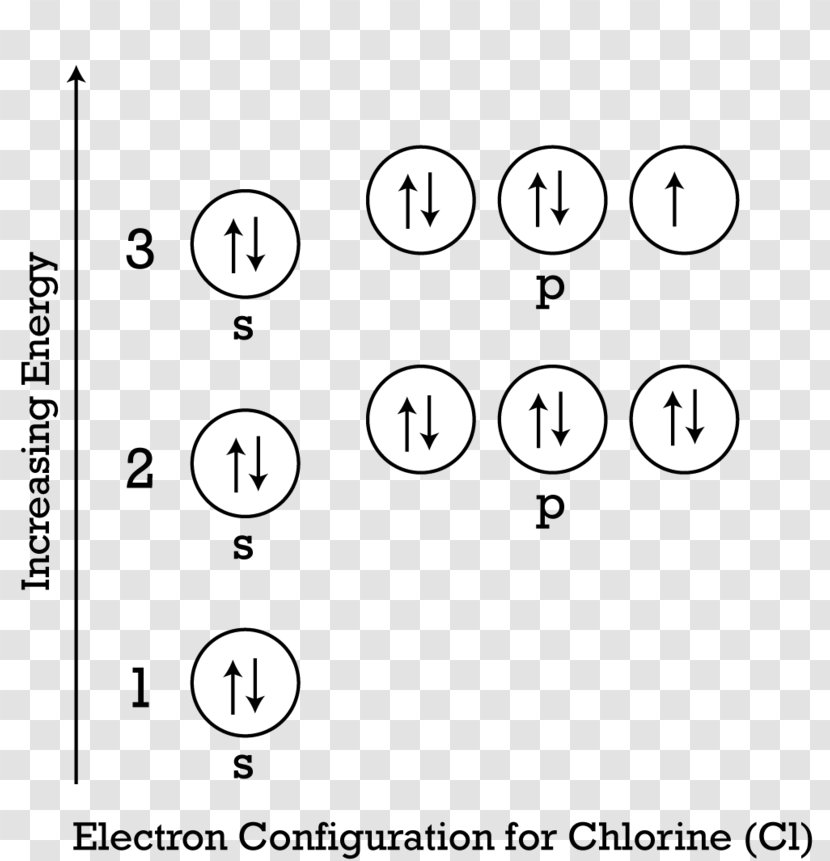
Version 2.0 and Part 7 of "Utopian Religion", "Utopia", or "Vispthinkingpat, Thinkflexsense, and Soundpat Religion" &#x200B; # Efficient vispthinkingpat combination of English language, numerical list format, and logic language or Vispenlogist Language: * Listology <-> List-types-ology <-> Indented-list-ology and Non-indented-list-ology and Numerically-ordered-list-ology and Bulletpoint-ordered-list-ology and Vispenlogistology * Combatology <-> Shield-from-enemy-ology... In writing the electron configuration for Chlorine the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Chlorine go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. I am doing a research project on 1.4 dichlorobenzene (C6H4Cl2) for grade 12 chemistry and I am stuck on the structural diagram. I know that it is a carbon ring with each carbon having a single bond with either the hydrogen or chlorine and that each carbon has a trigonal planar shape. Between the carbons, there will be alternating single and double bonds. I don't understand why the hybridization for the carbons is sp2 and not sp3 and why the third p orbital is at a right angle with the molecule a... The second member of the halogen family, chlorine is represented by Cl and has a total of 17 electrons among which two electrons belong to the K shell, eight to ...
Jan 06, 2020 · Atomic orbital diagram chlorine posted on december 23 2014 by admin shapes of p orbitals chemistry sodium orbital br diagram electron configuration group orbital diagrams an aufbau diagram showing the electron configuration of argon. The orbital diagram for its shells is 1s2 2s2 2p6 3s2 3p6 4s2 3d6. Construct the orbital diagram of each atom or ion. What would the orbital diagram for these molecules look like if they had this many electrons? &#x200B; I'd guessed that phosphorus would have 3s(2), 3p(3), and 3d(5). The half-filled p and d orbitals would offer stability to explain why phosphorus can sometimes make 5 bonds. Alternatively I was considering 3s(2), 3p(6), and 4s(2). &#x200B; I'd guessed that sulfur would have 3s(2), 3p(3), 3d(5), and 4s(2). Again, the half-filled p and d orbitals would offer stability. An electron from... 26 Jan 2021 — For writing the Chlorine Electron Configuration you first need to check the number of electrons for the Chlorine (Cl) atom (there are 17 ... Nov 14, 2011 · An orbital is a region of space that an electron can exist in. For the diagram you start with the 1 s orbital and then 2s, 2p, and so on. Each orbital can hold 2 electrons and each arrow ...
Hey, guys, Welcome back. All right, so in this practice problem, we are asked to draw a new atomic orbital diagram for chlorine.20 Feb 20181 answer · Top answer: Chlorine• Atomic # 17 : 17 electrons• Electron Configuration: 1s22s22p63s23p5 [readmore]Atomic Orbital Diagram• Lowest energy orbital at the bottom• ...
Solved Write The Full Electron Configuration Fill In The Orbital Diagrams And Determine The Magnetic Property For The Following Elements Nitrog Course Hero
Hey All, I'm new to the forum here and a struggling O-Chem student. I'm taking OChem 1, and although I do enjoy it, I'm still timid about my answers. I'm doing a test review problems, and I was wondering if I could get some help to verify my answers. I'm not hundred percent sure, and any inputs would be greatly appreciated. Anything that isn't clear, too vague, etc. Thank you in advance! :) (**4**). Explain why free radical halogenation produces racemic mixtures of products. -Because the ra...
Hey All, I'm new to the forum here and a struggling O-Chem student. I'm taking OChem 1, and although I do enjoy it, I'm still timid about my answers. I'm doing a test review problems, and I was wondering if I could get some help to verify my answers. I'm not hundred percent sure, and any inputs would be greatly appreciated. Anything that isn't clear, too vague, etc. Thank you in advance! :) (**4**). Explain why free radical halogenation produces racemic mixtures of products. -Because the ra...
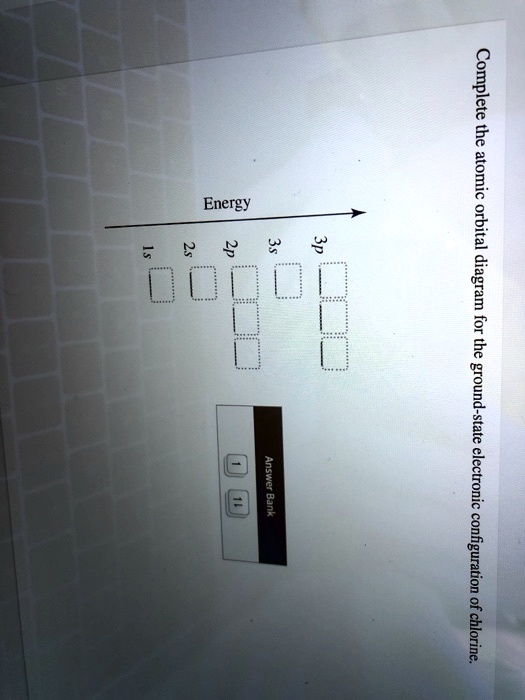
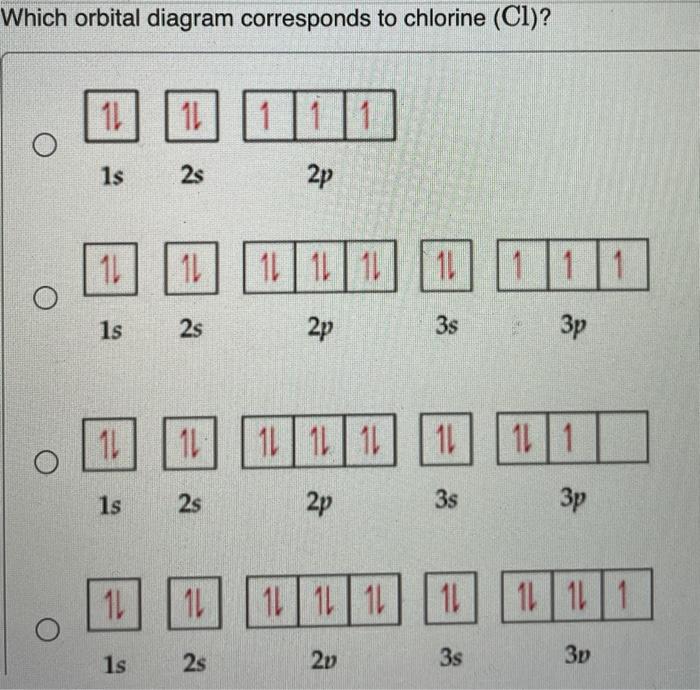
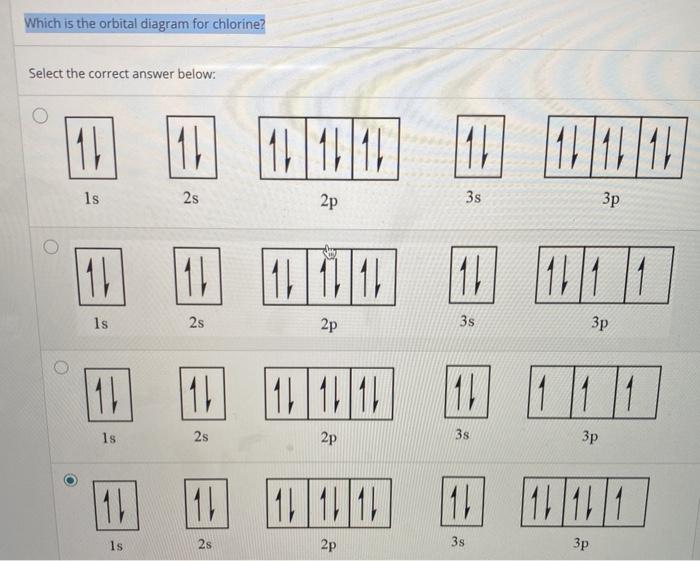
Solved Complete The Atomic Orbital Diagram For The Ground State Electronic Configuration Of Chlorine Energy 2p Answer Bank

Energetic Placement Of Atomic Orbitals In The Hcl Molecular Orbital Diagram Chemistry Stack Exchange

Create The Atomic Orbital Diagram For Chlorine In A Chlorine Atom Which Subshells Contain Valence Electrons Homeworklib
























Comments
Post a Comment