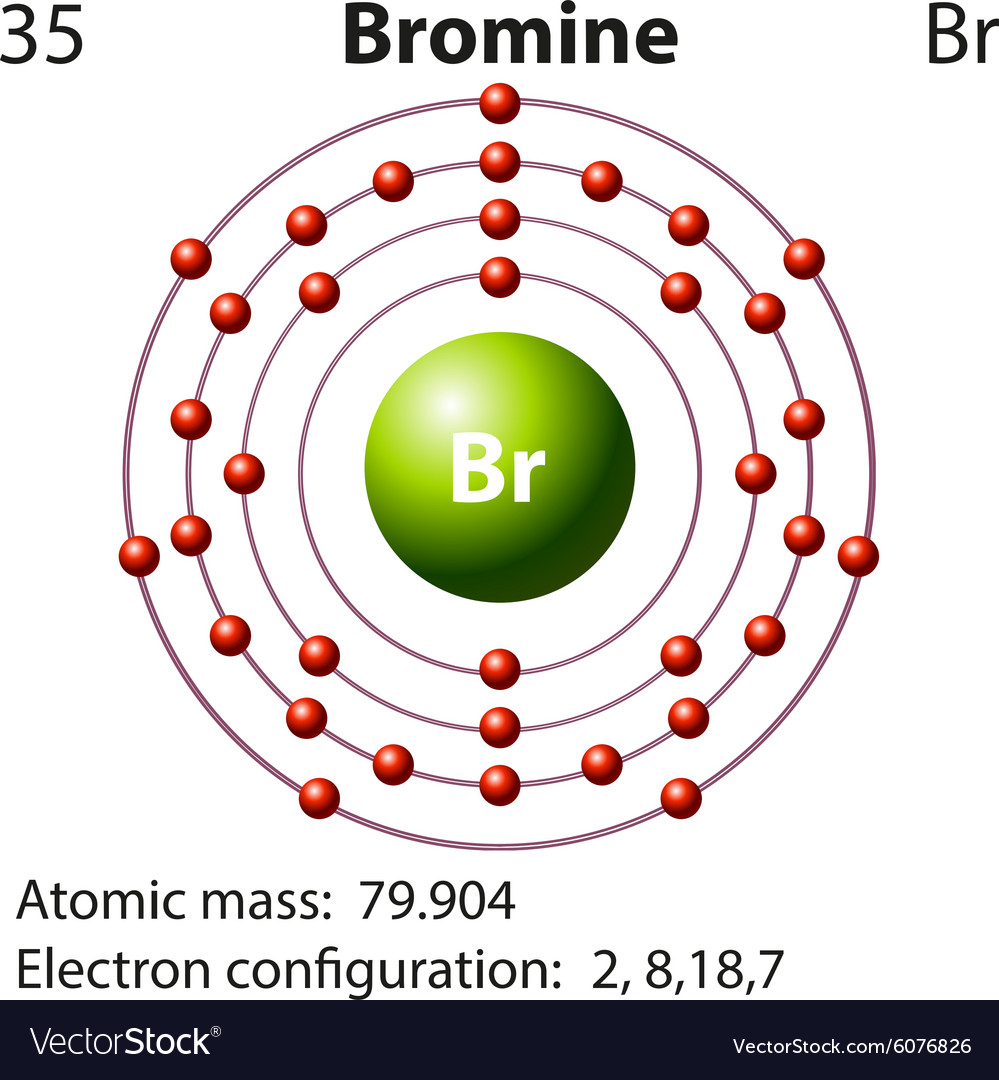
43 dot diagram for bromine
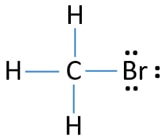
The lewis structure of bf3, boron trifluoride, has one boron atom in the centre, and three fluorine atoms surrounding it. It is a chemical formula for bromine trifluoride. Valence electrons bond pairs lone pairs sigma bonds pi bonds electron pair geometry . Sharing 1 electron per bond, bf3 is a stable compound. As the title states, I am an analytical guy attempting a bromine reaction. Specifically, I'll be reacting mixtures of [alkenes in fuel to obtain dibromides](https://www.masterorganicchemistry.com/reaction-guide/bromination-of-alkenes-with-br2-to-give-dibromides/). As someone who hasn't done an organic reaction since I took ochem 8 years ago, I have a couple questions: 1.) In previous literature refs I found (and these are analytical papers), the reaction is done in carbon tet. I'd like to av...
Use the tabs at the top of the Lewis Structure Drawing Tool to create correct expressions, For a detailed example, = Fluorine = Bromine = Chlorine A Lewis dot structure ofaround 'S' would have two dots on two sides, and one single on eachof the remaining. What is the Lewis diagram for fluorine? Lewis Structures and the Shapes of Molecules .

Dot diagram for bromine
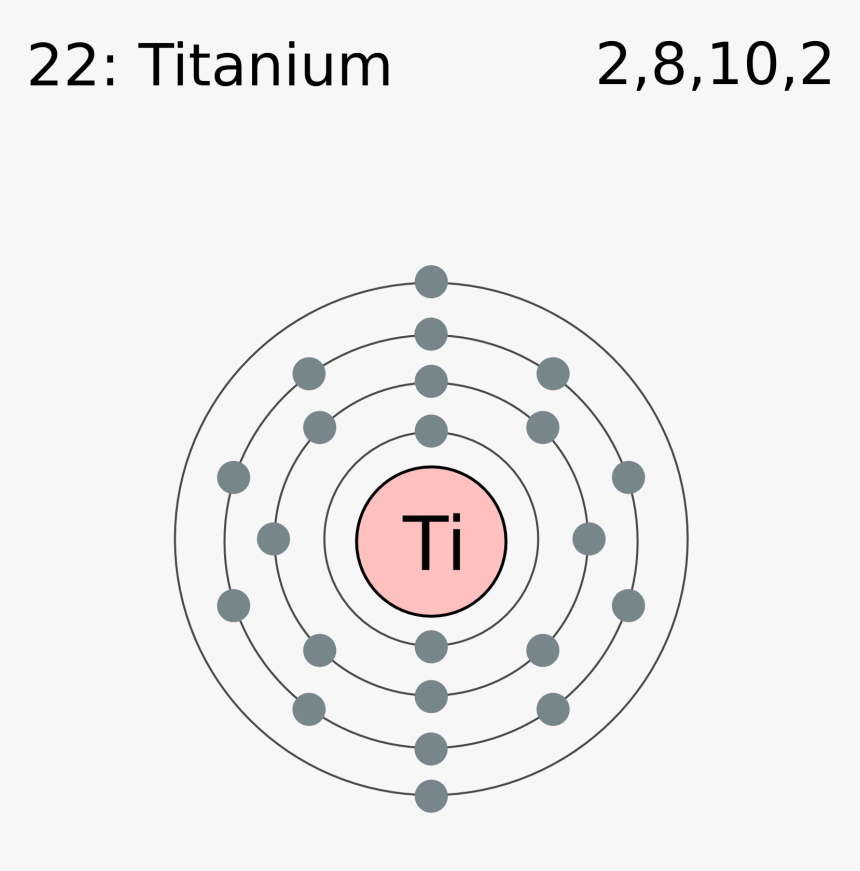
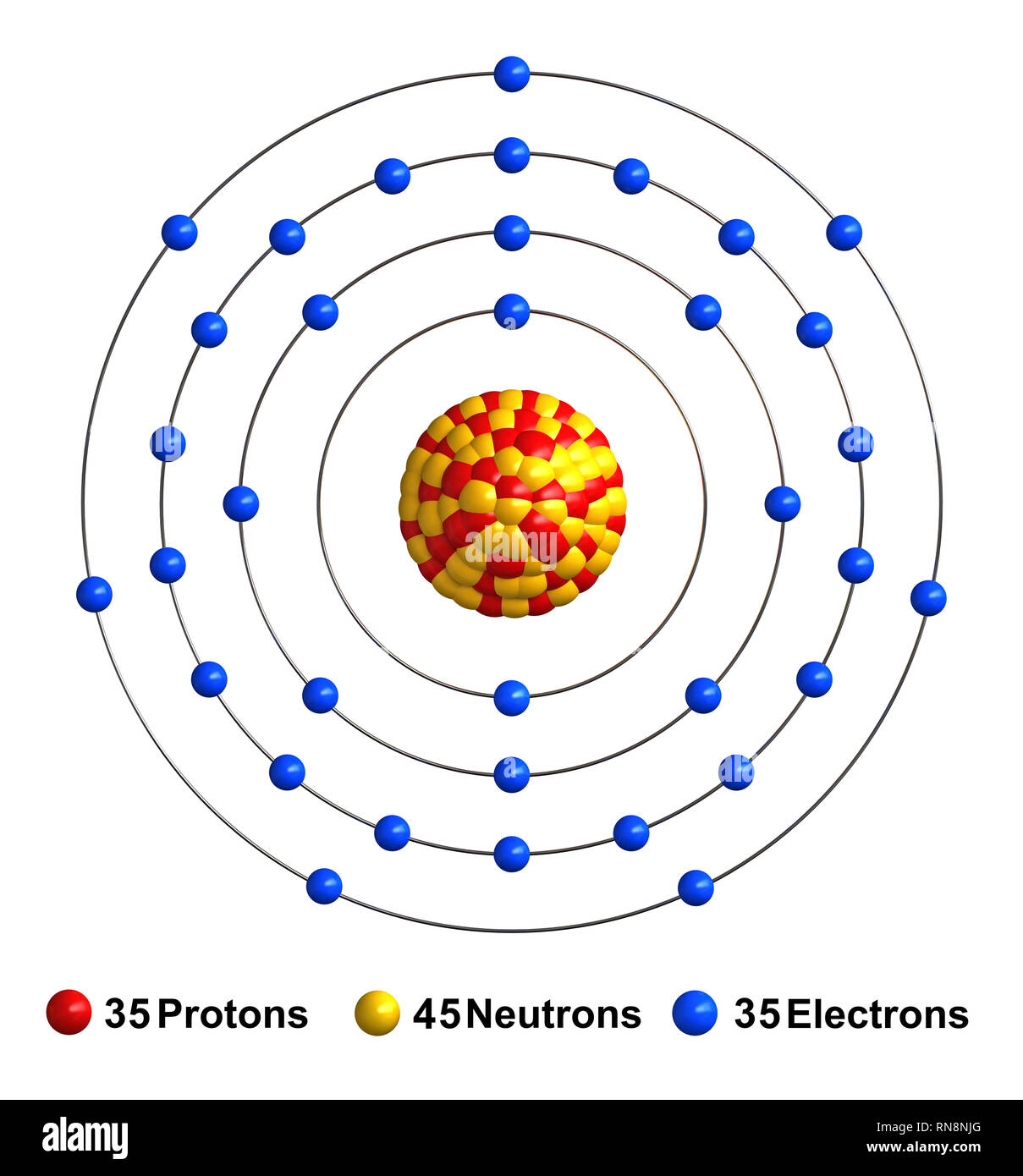
Once we know how many valence electrons there are in Br we can distribute them around the central atom with the goal of filling the outer shells ... Bromine Bohr Diagram. Here are a number of highest rated Bromine Bohr Diagram pictures on internet. We identified it from well-behaved source. Its submitted by giving out in the best field. We acknowledge this kind of Bromine Bohr Diagram graphic could possibly be the most trending topic subsequent to we share it in google gain or facebook. In the BrO3- Lewis structure Bromine (Br) is the least electronegative and goes in the center of the dot structure. Remember that Bromine (Br) can hold more ...
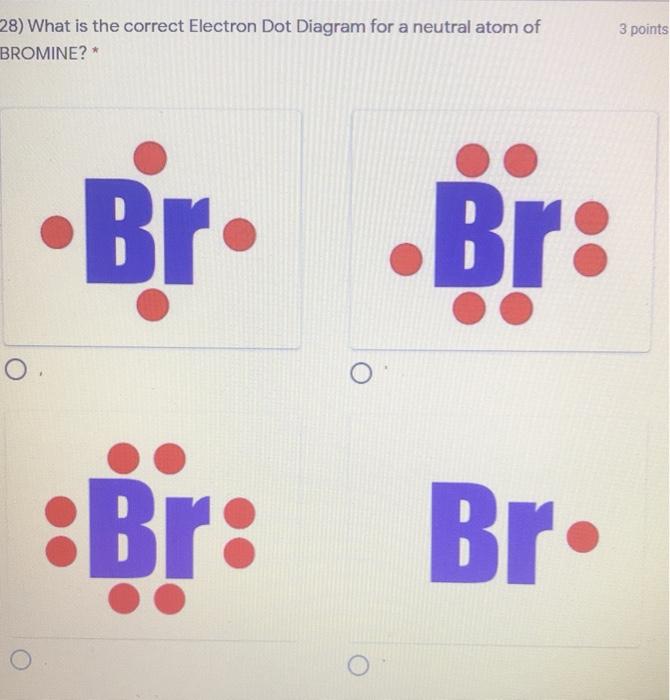
Dot diagram for bromine. PBr3 Lewis Structure, Molecular Geometry, Hybridization and Polarity. PBr3 is a chemical formula for Phosphorus Tribromide. The molecule is made up of one Phosphorus atom and three Bromine atoms. It is a colourless liquid with a pungent odour. Like PF3 and PCl3, PBr3 also exhibits the properties of both Lewis Acid and Lewis Base. Hello! Can someone please explain to me how to draw the Lewis diagrams for transition metals? I understand how to find the valence electrons based on the electron configuration. For elements 27+, they begin having more than 8 valence? Please help I am confused! Bromine has atomic number 35, which means it has 7 electrons in its valence shell. On the other hand, iodine is located in group 17 (main group 7), which means it has 7 valence electrons. Valence electrons can be counted using a Lewis electron dot diagram. … Because the chloride ion (Br-) has an extra electron (the negative sign denotes an extra electron) we need to add that to the 7 valence ...
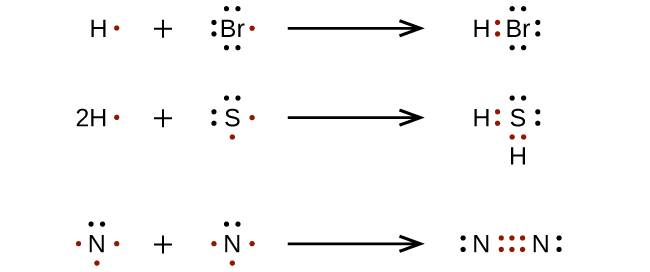
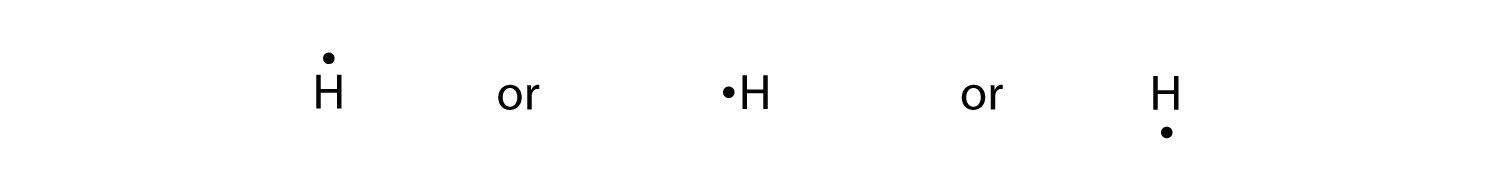
Viewing Notes: The Lewis structure for SO 2 requires you to place more than 8 valence electrons on Sulfur (S).; You might think you've got the correct Lewis structure for SO 2 at first. Lewis structures extend the concept of the electron dot diagram by adding lines between atoms to represent shared pairs in a chemical bond. H - Br There should be one dot to the right of the Hydrogen atom (H), sharing a bond with a dot to the left of the Bromine atom (Br). Similar arguments can be applied to boron trichloride, BCl 3, which is a stable gas at room temperature. Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13: Orbital diagram of Aluminum (Al) 14: Orbital diagram of Silicon (Si) 15 ... BrF5 lewis period structure has actually 1 bromine and also 5 fluorine atom. Over there is one lone pair current on bromine and also it is associated with 5 fluorine atoms v the assist of five solitary bonds. Follow some steps for drawing the lewis dot framework of BrF5. 1. Count full valence electron in BrF5
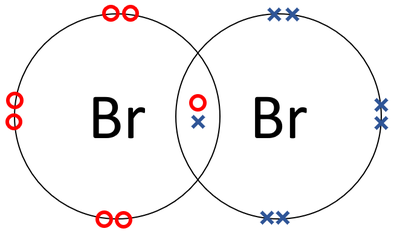
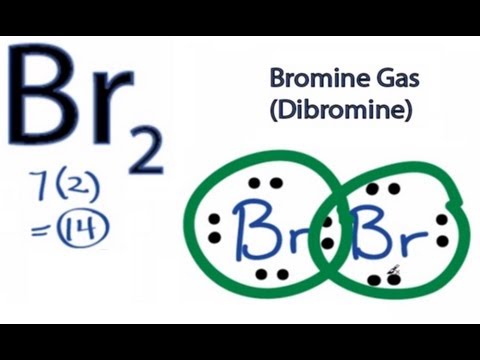
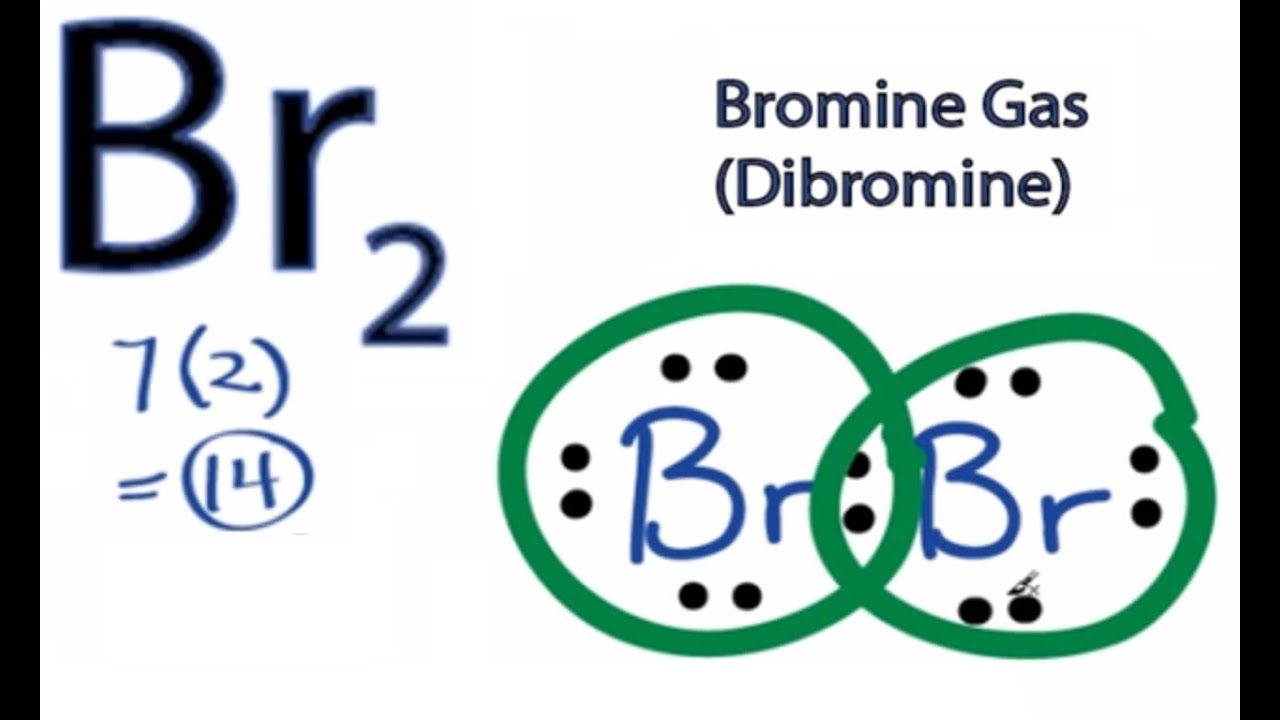
How to Draw the Lewis Dot Structure for Br2 : Diatomic Bromine. Related Searches. br2 bond type polar or nonpolar covalent bond of br2 type of chemical bond present in i2 br2 and cl2 o2 bond order bond order of n2 f2 bond order bond order formula bond energy of i2. See more articles in category: FAQ. Lewis Structure. The Lewis structure or electron dot structure is a diagram that represents the single pair and bonded pair of the atoms in the molecule hybridization. The single bond is shown using lines with bond angle, and the lone pairs are presented using dots. ... With this, Bromine can use the D-Orbitals for hybridization that is useful ... Bromine has seven , all located on the fourth energy level in the 4s and 4p-obitals. This means that its electron dot diagram will feature its chemical symbol surrounded by seven dots When potassium and bromine come together, the more bromine will snatch that solitary valence electron from the potassium atom. Transcript: This is the Br2 Lewis structure. Looking on the periodic table, Bromine is in Group 7 or 17. It has 7 valence electrons. We have two of them though.
A step-by-step explanation of how to draw the Br2 Lewis Dot Structure (Bromine gas).For the Br2 structure use the periodic table to find the ...
Bromine Valence Electrons. Here are a number of highest rated Bromine Valence Electrons pictures upon internet. We identified it from obedient source. Its submitted by supervision in the best field. We take on this kind of Bromine Valence Electrons graphic could possibly be the most trending subject once we part it in google benefit or facebook.
Bromine Electron Dot Diagram ... Since Bromine is in group, or series, 17, it has 7 valence electrons. Group 1 on the periodic table has 1 valence electron. Group ...
Lewis Dot Structure For Hbr - There is a single bond connecting hydrogen and bromine. Calculation of total valence electron of CO2 molecule Choose the atom with the least electronegative value atom and insert it in the center of the molecular geometry of CO2.
for the bro2 - structure use the periodic table to find thelewis structures, also known as lewis dot formulas, lewis dot structures, electron dot structures, or lewis electron dot structures (leds), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule.from the …
What is the Lewis structure of Cl2? For example, when two chlorine atoms, each with 7 valence electrons, come together to form a diatomic chlorine molecule, the Lewis structure shows that there will be a sharing of two electrons between the two chlorine atoms which allows both chlorine to be surrounded by 8 electrons….Lewis Dot Structures.
Lewis Structure The Lewis framework or electron dot framework is a diagram the represents the solitary pair and bonded pair of the atom in the molecule hybridization. The solitary bond is shown using lines with bond angle, and also the lone pairs room presented using dots. ... Bromine have the right to use the D-Orbitals for hybridization the ...
17.10.2015 ... Here's how that would look. Explanation: I'm not really sure if you're interested in the electron dot diagram of the potassium and bromine ...
Carbon Electron Configuration: If you guys have come across our recent article then it would be easy for you all to understand the concept. But if you are new here and looking for the information related to the carbon element and its electronic configuration, then today we will help you with some of the things and if you will be here till the last line surely you will go with …
17.02.2018 ... What is the lewis structure for hcn? How is vsepr used to classify molecules? What are the units used for the ideal gas law? How does Charle's ...
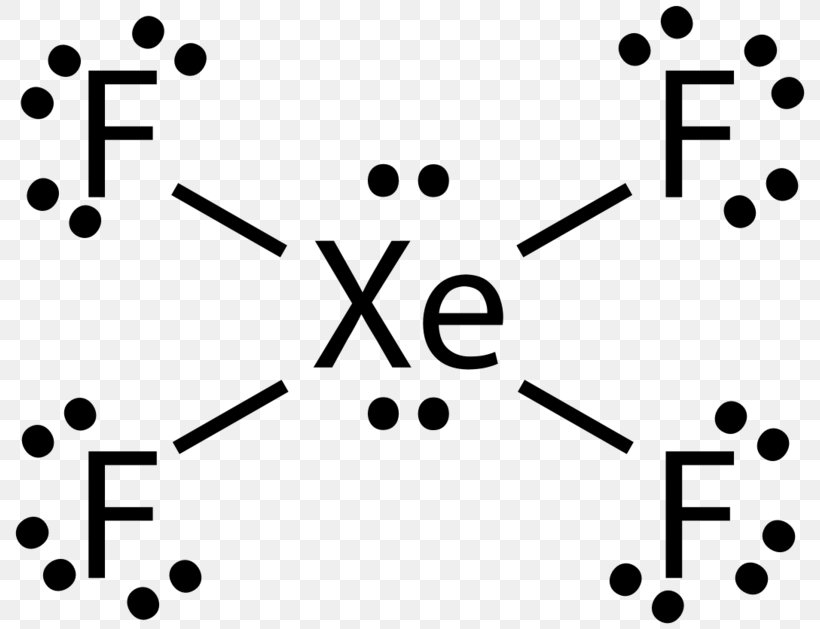
BrF3 Lewis Structure (Bromine Trifluoride) Watch later Watch on A Lewis dot structure or electron dot structure is a diagram that shows the bondings of the atoms in the molecule along with their lone pairs. The bonds in the diagram are shown by using lines, whereas the lone pairs are represented as dots.
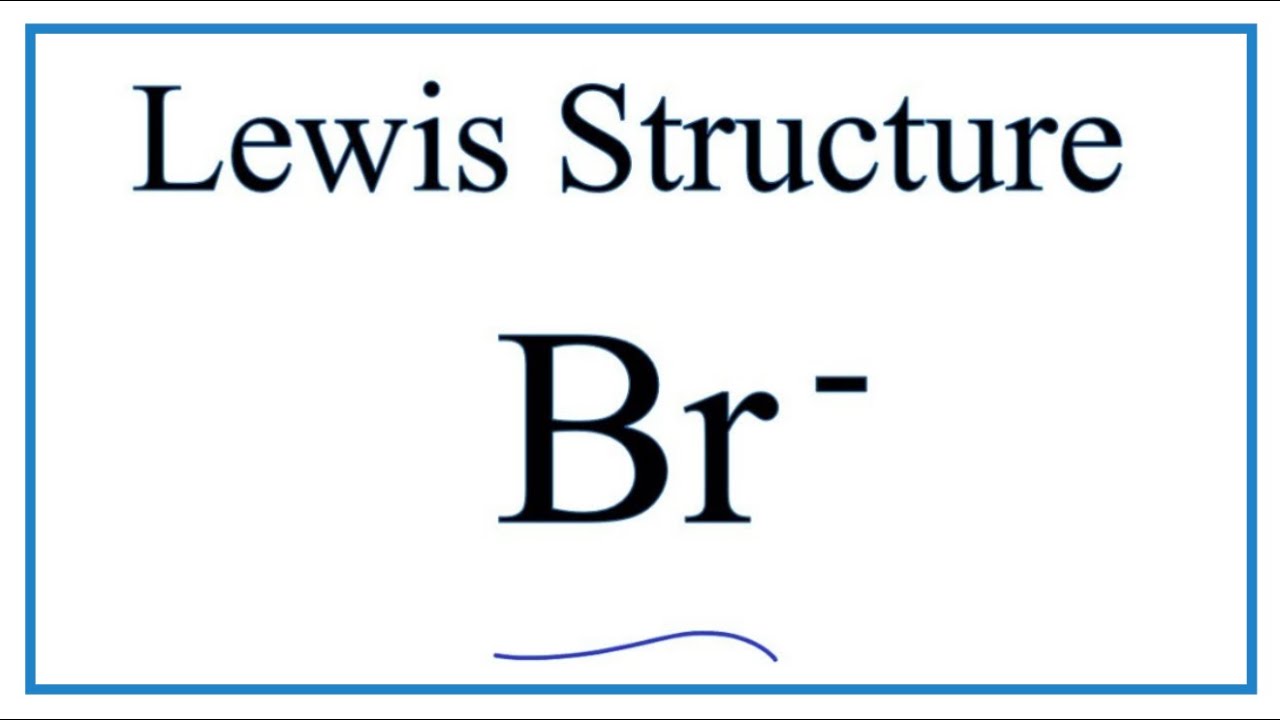
10. Draw the Lewis electron dot diagram for each ion. a) In +. b) Br −. 11. Draw ...
Today in class my professor was going over how to determine the rates of chlorination/bromination. He discussed how chlorination was less uphill and much faster because of lower activation energy due to its higher electronegativity, whereas bromination is way more uphill and much slower because of its higher activation energy due to its lower electronegativity. This part, I understood. However, my confusion began with the "uphill-ness" of the overall reaction compared to that of the transition ...
To draw the Lewis electron dot diagram we picture in our minds the symbol for Mg in a box with all of its core electrons (i.e., 1s22s22p6). Then we place the valence electrons around the sides of the box with each side representing an orbital in the outermost energy level. ... Bromine is used in many areas such as agricultural chemicals ...
In Section 9.1 "Lewis Electron Dot Diagrams," we saw how ions are formed by losing electrons to make cations or by gaining electrons to form anions. aluminum oxide aluminum oxide lewis dot structure. 7 thoughts on " Lewis dot diagram for rubidium " Sbg_ofp says: 05.02.2019 at 11:28.
As we know that the carbon has '4' valence electrons, bromine has '7' valence electron. Therefore, the total number of valence electrons in = 4 + 4(7) = 32. According to Lewis-dot structure, there are 8 number of bonding electrons present between the carbon-bromine and 24 number of non-bonding electrons present on the bromine.
Lewis Structure Hbr - Remember to include all of the electrons and any formal charges. 93 438 ratings Problem Details.. How To Determine The Lewis Dot Structure For Hydrogen Bromide Hbr Quora . Draw the Lewis structure for HBr. Hydrogen has only one electronic shell with one electron and needs only two electrons to achieve octet fulfillment.
As we all know the atomic number of bromine is 35 and it is one of the halogen gas. The electronic configuration of bromine is...
There are 24 valence electrons in total. 4 from carbon (black dots) and 3 x 6 from oxygen (red dots), making 22 valence electrons plus an extra 2 (blue dots) ...
In the BrO3- Lewis structure Bromine (Br) is the least electronegative and goes in the center of the dot structure. Remember that Bromine (Br) can hold more ...
Bromine Bohr Diagram. Here are a number of highest rated Bromine Bohr Diagram pictures on internet. We identified it from well-behaved source. Its submitted by giving out in the best field. We acknowledge this kind of Bromine Bohr Diagram graphic could possibly be the most trending topic subsequent to we share it in google gain or facebook.
Once we know how many valence electrons there are in Br we can distribute them around the central atom with the goal of filling the outer shells ...












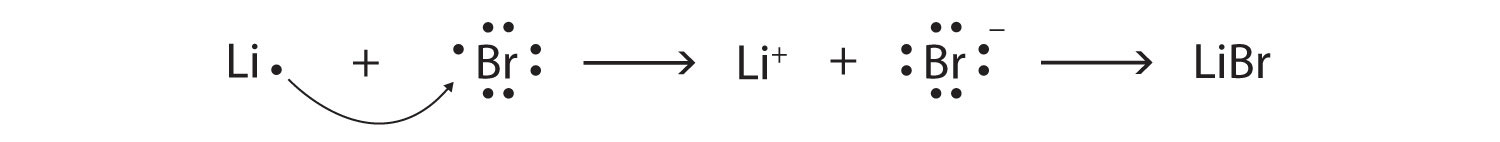











Comments
Post a Comment