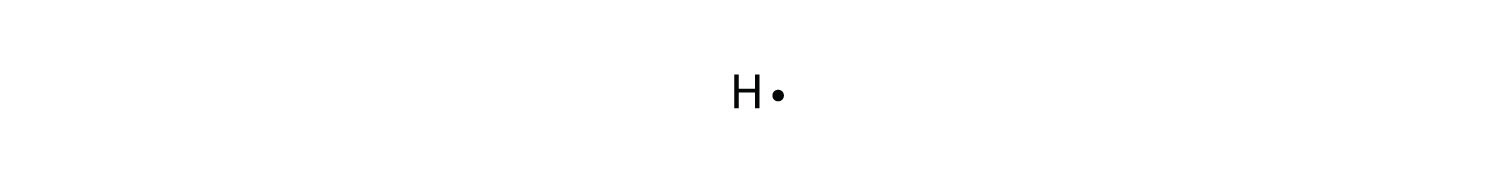
42 lewis dot diagram for h+
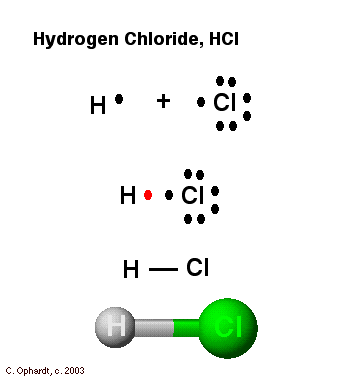
chem Flashcards - Quizlet The Lewis dot structure is used to keep track of the valence electrons for each atom. Give the symbol of the element in Group 6A, Period 3. S. Write the symbol for the element with the following electron configuration? 1s22s22p63s23p1. Al. Write the symbol for the element with the following electron configuration. [Kr]4s23d6. Fe. Match the type of … PDF How Atoms Combine - Lewis Diagrams, Ionic & Covalent Bonding Lewis diagram. H+ f. Hf. Notice that when H and F share electrons that each is able to complete its outer shell. Q7 - Draw Lewis dot diagrams to represent the following compounds (think about whether they are ionic or covalent): CCl4, HCl, MgF2, H2O, NH3, NaCl, OH-, H2.
Lewis Dot Diagram For Nitrogen - Free Wiring Diagram Lewis electron dot diagrams for ions have fewer for cations or more for anions dots than the corresponding atom. The lewis dot structure for nh3 ammonia is shown above. The location of the double bond changes over time meaning that at any point either of the oxygen atoms could have a...
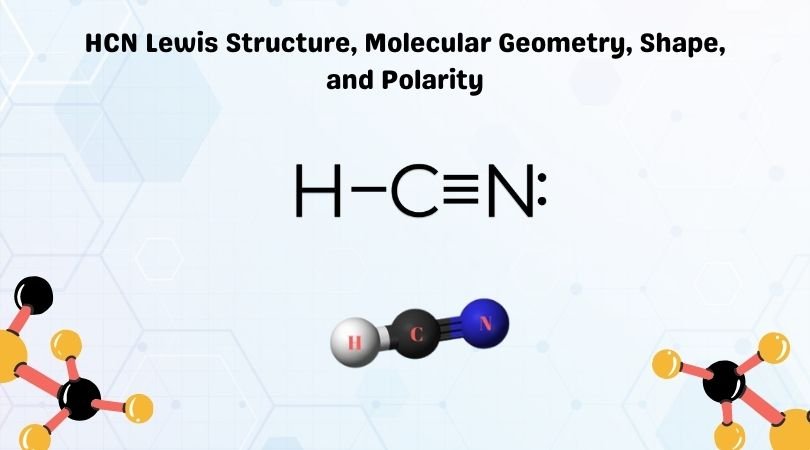
Lewis dot diagram for h+
Lewis dot diagrams 3. What are Lewis Dot Diagrams • Lewis dot diagrams show the localization of valence electrons - to which atoms the electrons belong. • There are a few more rules for drawing dot diagrams for molecules. Follow the rules and the dot diagrams should not be too difficult. Lewis Dot Diagram - Organic Chemistry | Socratic Lewis dot diagrams are a shorthand depiction of the bonds between several atoms and any unbonded electron pairs. Lines connect atoms to depict bonding and The Lewis dot diagram for the covalent bonding of chlorine, ( [Math Processing Error]. ), would be: When atoms are bonded ionically, the... PDF Lewis Dot Diagrams & Structures Lewis Dot Diagrams & Structures. General Instructions: Please do the activities for each day as indicated. Any additional paper needed please attach. Lewis Dot Diagrams & Structures. How are electrons shared to create covalently bonded molecules?
Lewis dot diagram for h+. Lewis Dot Diagrams of the Elements Lewis Dot Diagrams of Selected Elements. Lewis Symbols. Electron Configuration into Shells. PDF Lewis dot diagrams (structures) for atoms and ions predicting... Draw Lewis dot structures for each of the following atoms: Aluminum. Determine the common oxidation number (charge) for each of the following ions, and then draw their Lewis Dot Structure. Don't forget to show brackets and charge on your LDS for ions! Bohr-Rutherford Diagrams & Lewis Dot Diagrams - Eve... Lewis Dot Diagrams When we drew atomic models, you saw that for each atom concentric circles were used to represent energy levels and dots for electrons. A Lewis dot structure is like a simplified Bohr-Rutherford model. The Lewis Dot diagram contains the element symbol with dots representing... Lewis Dot Diagrams for Compounds - ppt download Draw Lewis Dot Diagrams for molecular compounds. How would we draw the Lewis dot diagram for oxygen (O), which has the electron configuration of 1s2 2s2 2p4 ? The p-electrons (there are 4 in our example) are placed one to a side, until they must double up.
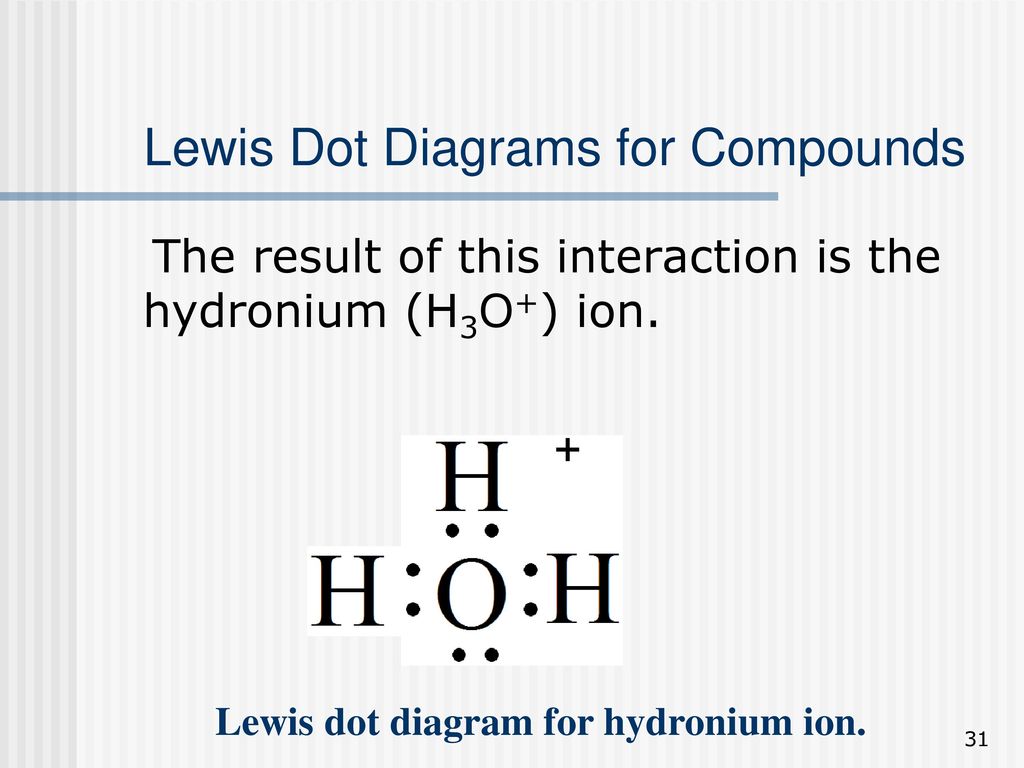
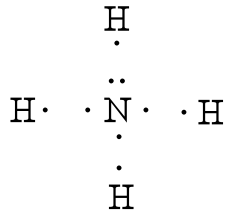
› 49212961 › Inorganic_Chemistry_by(PDF) Inorganic Chemistry by Miessler ~ 5th Edition | Arnab ... This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry. › 2020 › 11NH2- Lewis Structure, Molecular Geometry, Polarity ... Nov 10, 2020 · It has a total of 8 valence electrons which are participated in the formation of the Lewis dot structure whereas there are 2 bonding pairs and 2 lone pairs of electrons within the molecule. Due to the presence of two lone pairs of electrons that repel bond pairs N-H, it acquires a bent V-shape molecular shape with a bond angle of 104.5 ° . PDF Lewis Dot Diagrams 1 Guidelines to constructing lewis dot diagrams. [1] Determine which atoms are attached to each other. [a] The central atom of a molecule is often written first in the formula, followed by the atoms that surround it (e.g., CO2, NH3, NO2, NO3−, SO3, CO32−, SO42− H+ HNH. Lewis Electron Dot Diagrams - Help A Lewis Structure is a diagram that shows how valence electrons within a compound are distributed among its atoms. Those electrons that are shared by two Those electons that are located on a single atom are referred to as lone pairs and represented by two dots. Thinking About Lewis Structures.
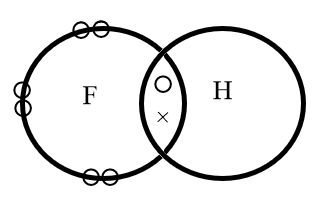
chemistry-lewis dot diagrams A Lewis structure or Lewis dot diagram, represents the bonds formed between two non-metal atoms as they share electrons. These diagrams show only the valence electrons of each atom as they are distributed amongst the bonded atoms. Drawing such diagrams is a great start to understanding how... NO Lewis Dot Structure | Science Trends A Lewis structure (also called Lewis dot formulas, Lewis dot structures, or electron dot structures) are pictorial diagrams that represent the bonding Lewis diagrams contain 3 basic elements: symbols that represent individual atoms, dots that represent electrons, and unbroken lines that represent... Solved Draw the Lewis dot diagram for a H+ cation Make Lewis Structures (electron dot diagrams) Lewis Structures of Atoms The gained or lost 1e lost Example Lewis Structure (electron dot diagram) H+ 2+ 3+ 2e lost Li2O Each 8 hours ago Lewis dot diagram for h2s. Plus sulfur is in group 6 or 16 on the periodic table so it has 6 valence electrons. AP Chemistry Lab Manual - PC\|MAC Prepare the reaction chamber according to the following diagram. You will need the 250mL beaker, the penny, the copper wire, the wire mesh, and a ruler. The penny is to be suspended 2cm above the bottom of the beaker. The copper wire is directed thru the wire mesh and bent at a 90o angle to hold the penny in the proper location. Remove the wire mesh (with the penny and …
quizlet.com › 556589717 › mid-term-chem-flash-cardsMid Term Chem Flashcards | Quizlet Lewis electron-dot diagrams for CO2 and SO2 are given above. The molecular geometry and polarity of the two substances are A. the same because the molecular formulas are similar B. the same because C and S have similar electronegativity values C. different because the lone pair of electrons on the S atom make it the negative end of a dipole
Как изобразить точечные структуры Льюиса - wikiHow /thumb\/a\/aa\/Draw-Lewis-Dot-Structures-Step-2-Version-4.jpg\/v4-728px-Draw-Lewis-Dot-Structures-Step-2-Version-4.jpg","smallWidth":460,"smallHeight":306,"bigWidth":728,"bigHeight":485,"licensing":"
Lewis Structures: Learn How to Draw Lewis Structures | Albert.io A Lewis Dot Structure is drawn by a series of dots, lines, and atomic symbols and provides a structure for the way that the atom or molecule is arranged. A Lewis Dot Structure can be made for a single atom, a covalent compound, or a polyatomic ion.
9.2: Lewis Electron Dot Diagrams - Chemistry LibreTexts Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Draw a Lewis electron dot diagram for an atom or a monatomic ion. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms.
phet.colorado.eduPhET: Free online physics, chemistry, biology, earth science ... Founded in 2002 by Nobel Laureate Carl Wieman, the PhET Interactive Simulations project at the University of Colorado Boulder creates free interactive math and science simulations. PhET sims are based on extensive education research and engage students through an intuitive, game-like environment where students learn through exploration and discovery.
h lewis dot structure - Search Lewis Structures (electron dot diagrams) Lewis Structures of Atoms The gained or lost 1e lost Example Lewis Structure (electron dot diagram) H+ 2+ 3+ 2e lost Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule.
Lewis Structures or Electron Dot Structures Diagraming elements of molecular structure. Lewis structures, also known as electron dot structures, are named after Gilbert N. Lewis, who described them in a 1916 article titled, "The Atom and the You can draw a Lewis dot structure for any covalent molecule or coordination compound.
Lewis Dot Diagram For H+ Cation Lewis Structures (electron dot diagrams) Lewis Structures of Atoms The gained or lost 1e lost Example Lewis Structure (electron dot diagram) H+ 2+ 3+ 2e lost Li2O Each lithium atom loses one electron to form 2 cations Li+ (2 electrons in.
6.1 Lewis Electron Dot Diagrams | Introductory Chemistry 6.1 Lewis Electron Dot Diagrams. Learning Objectives. By the end of this section, you will be able to A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of...
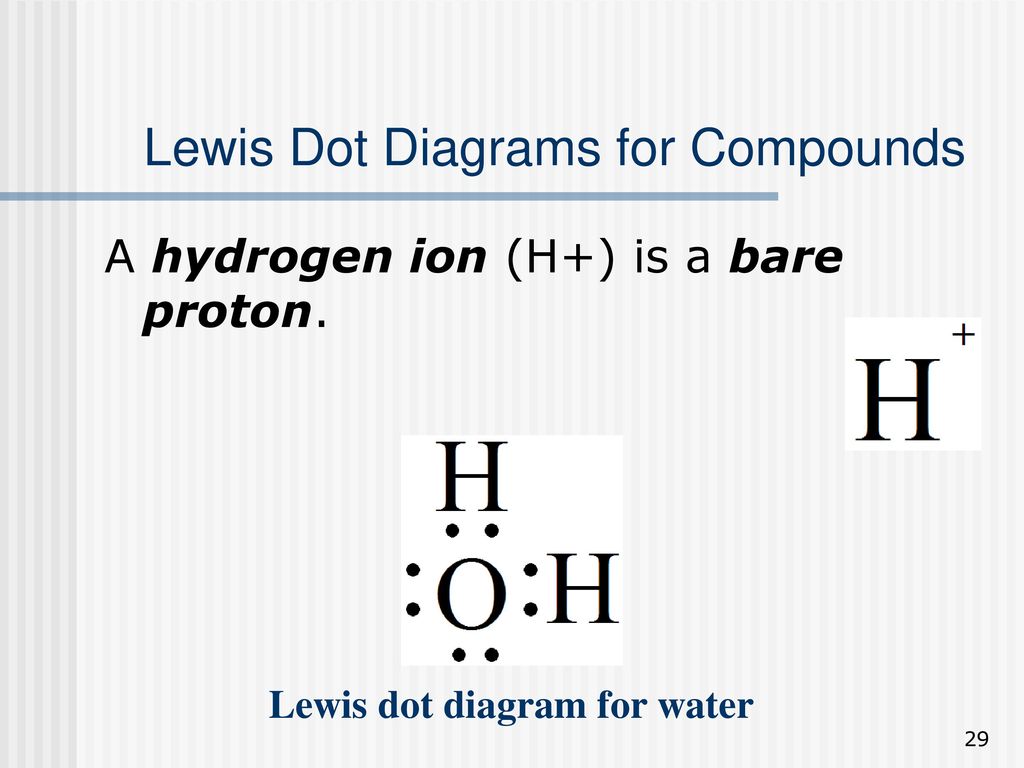
How would you show the bonding of Hydrogen and Oxygen ... 7 Mar 2017 — And thus we have to distribute 8 electrons in the Lewis dot diagram. pic2fly.com. Of course, the electronic geometry is tetrahedral that ...1 answer · There are 2 valence electrons from the hydrogen atoms, and 6 valence electrons from the oxygen atom.......... Explanation: And thus we have to distribute ...
How to find the Lewis dot diagram for H2O - Quora How do you find the Lewis dot diagram for H2O? A Lewis structure is a diagram that shows the bonding between the atoms of a molecule and any possible lone pairs of electrons.
quizlet.com › 604727995 › acellus-general-chemistryACELLUS GENERAL CHEMISTRY Flashcards | Quizlet Based on the Lewis/electron dot representation of the two atoms, predict the ratio of metal cationic (+) atom to nonmetal anionic (-) atom in the compound. Al O 2:3
Lewis Electron Dot Diagrams Draw a Lewis electron dot diagram for an atom or a monatomic ion. In almost all cases, chemical bonds are formed by interactions of valence electrons For example, the electron dot diagram for iron (valence shell configuration 4s23d6) is as follows: Elements in the same column of the periodic table...
Lewis Structures (electron dot diagrams) Charge on Ion 1+ No. electrons gained or lost 1e lost Example Lewis Structure (electron dot diagram) H+ 2+ 3+ 2e lost 3e lost 4e lost Group Group II 2+ I+ Group (alkali (Alkali III 3+ earth metals) metals) OR H+ OR Li+ 4+ OR 4-2-4e 3e 2e gained gained gained Group Group IV.
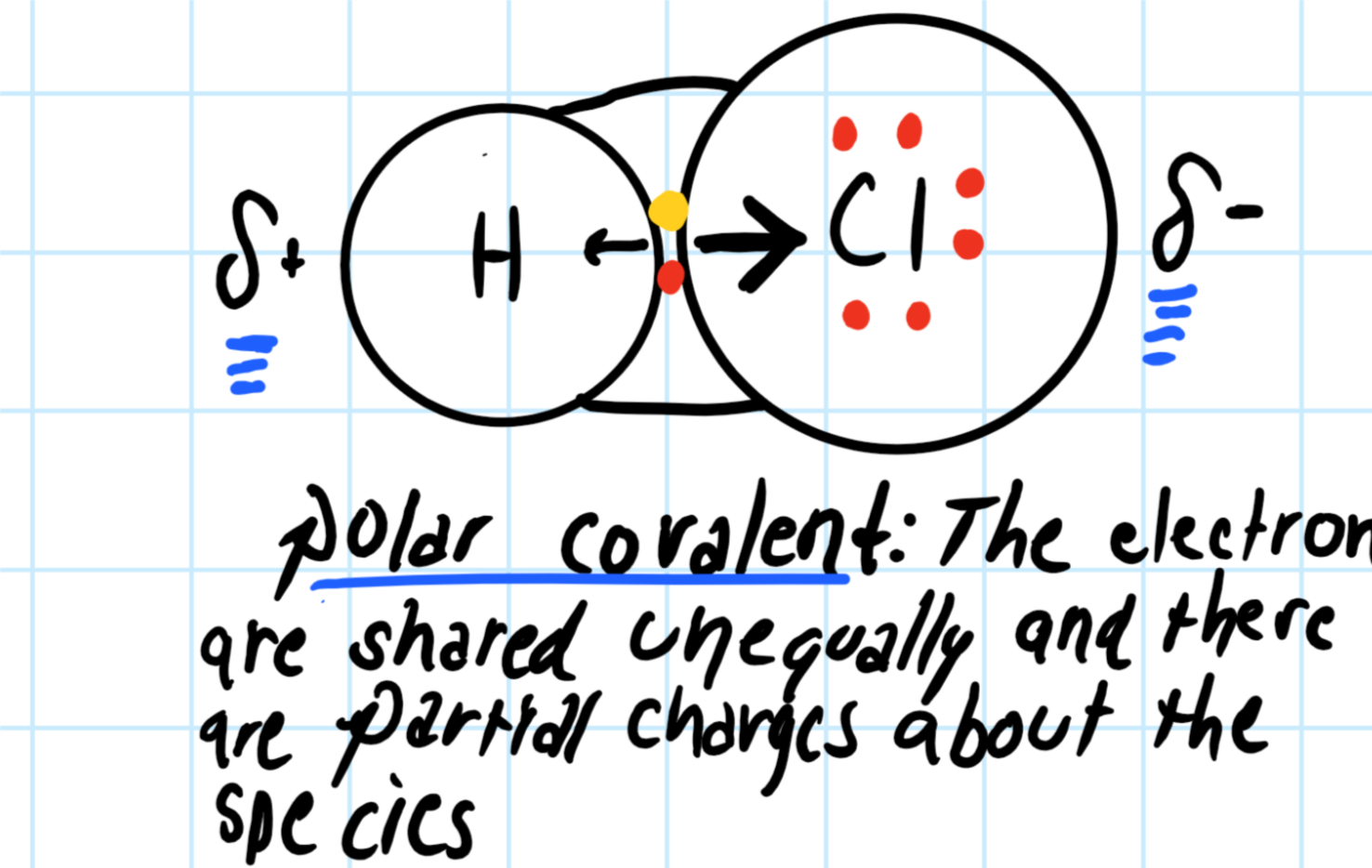
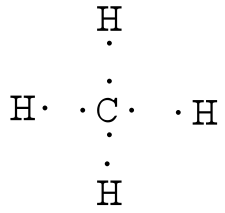
en.wikipedia.org › wiki › Covalent_bondCovalent bond - Wikipedia Lewis proposed that an atom forms enough covalent bonds to form a full (or closed) outer electron shell. In the diagram of methane shown here, the carbon atom has a valence of four and is, therefore, surrounded by eight electrons (the octet rule ), four from the carbon itself and four from the hydrogens bonded to it.
Lewis Electron Dot Diagram - Concept - Chemistry Video by Brightstorm Lewis Dot Diagrams are used to visually depict bonding by representing valence electrons as dots surrounding an elemental symbol. Lewis Dot Diagrams are illustrations of how the elements in a covalent bond come together to form a new, a structure or a molecule.
Lewis Structures (electron dot diagrams) Chemistry Tutorial Lewis Structures or electron dot diagrams for atoms, ions, ionic compounds and covalent compounds tutorial with worked examples for chemistry students. Electrons in the Lewis Structure (electron dot diagram) are paired to show the bonding pair of electrons.
Organic Chemistry 4th ed - Paula Bruice - Academia.edu Academia.edu is a platform for academics to share research papers.
Lewis Dot Diagrams of the Elements - STEM Sheets Lewis dot diagrams are a visual representation of the valence electrons on an atom of each individual element. These diagrams are used to draw Lewis dot structures, also know as electron dot structures or Lewis dot formulas, of compounds.
How to Draw the Lewis Dot Structure for H+ (Hydrogen ion) - YouTube A step-by-step explanation of how to draw the H+ Lewis Dot Structure.For the H+ structure use the periodic table to find the total number of valence...
Lewis structure - Wikipedia Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule.
varazze5stelle.it 23.02.2022 · Lewis electron-dot diagrams and sketches of molecules may be helpful as part of your explanations. 14 g of CO 2 and 1. Which of the following is a correct Lewis structure for oxygen? trigonal-pyramidal molecular geometry 4. Students will learn to calculate empirical and molecular formulas and practice applying logical problem- AP Chemistry: solutions follow. 7% …
High School Chemistry/Lewis Electron Dot Diagrams - Wikibooks... This chapter will explore yet another shorthand method of representing the valence electrons. The method explored in this lesson will be a visual representation of the valence electrons. We will, as we observed in the previous lesson...
chemdictionary.org › dative-bondDative Bond | Definition, Examples, How To Identify Oct 22, 2019 · Lewis Dot Diagram of AlCl 3. Aluminium chloride is an electron-deficient. There is a similarity between AlCl 3 and BF 3 because aluminium and boron belong to the same group of the periodic table. The measurements of the relative formula mass of aluminium chloride describe that its formula in the vapor at the sublimation temperature is not AlCl ...
(PDF) Inorganic Chemistry Housecroft | Yurika Almanda ... Pembuatan Senyawa kompleks asetial asetanoat. Enter the email address you signed up with and we'll email you a reset link.
PDF Lewis Dot Diagrams & Structures Lewis Dot Diagrams & Structures. General Instructions: Please do the activities for each day as indicated. Any additional paper needed please attach. Lewis Dot Diagrams & Structures. How are electrons shared to create covalently bonded molecules?
Lewis Dot Diagram - Organic Chemistry | Socratic Lewis dot diagrams are a shorthand depiction of the bonds between several atoms and any unbonded electron pairs. Lines connect atoms to depict bonding and The Lewis dot diagram for the covalent bonding of chlorine, ( [Math Processing Error]. ), would be: When atoms are bonded ionically, the...
Lewis dot diagrams 3. What are Lewis Dot Diagrams • Lewis dot diagrams show the localization of valence electrons - to which atoms the electrons belong. • There are a few more rules for drawing dot diagrams for molecules. Follow the rules and the dot diagrams should not be too difficult.

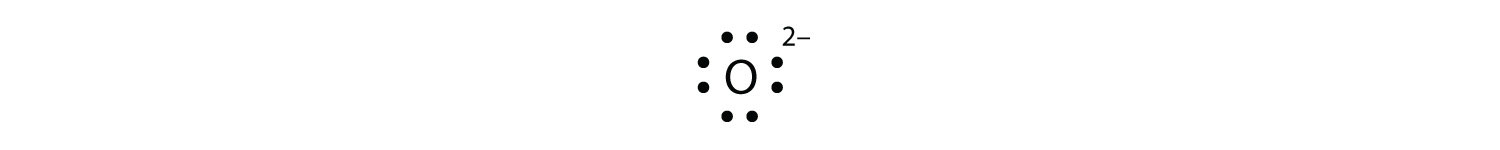

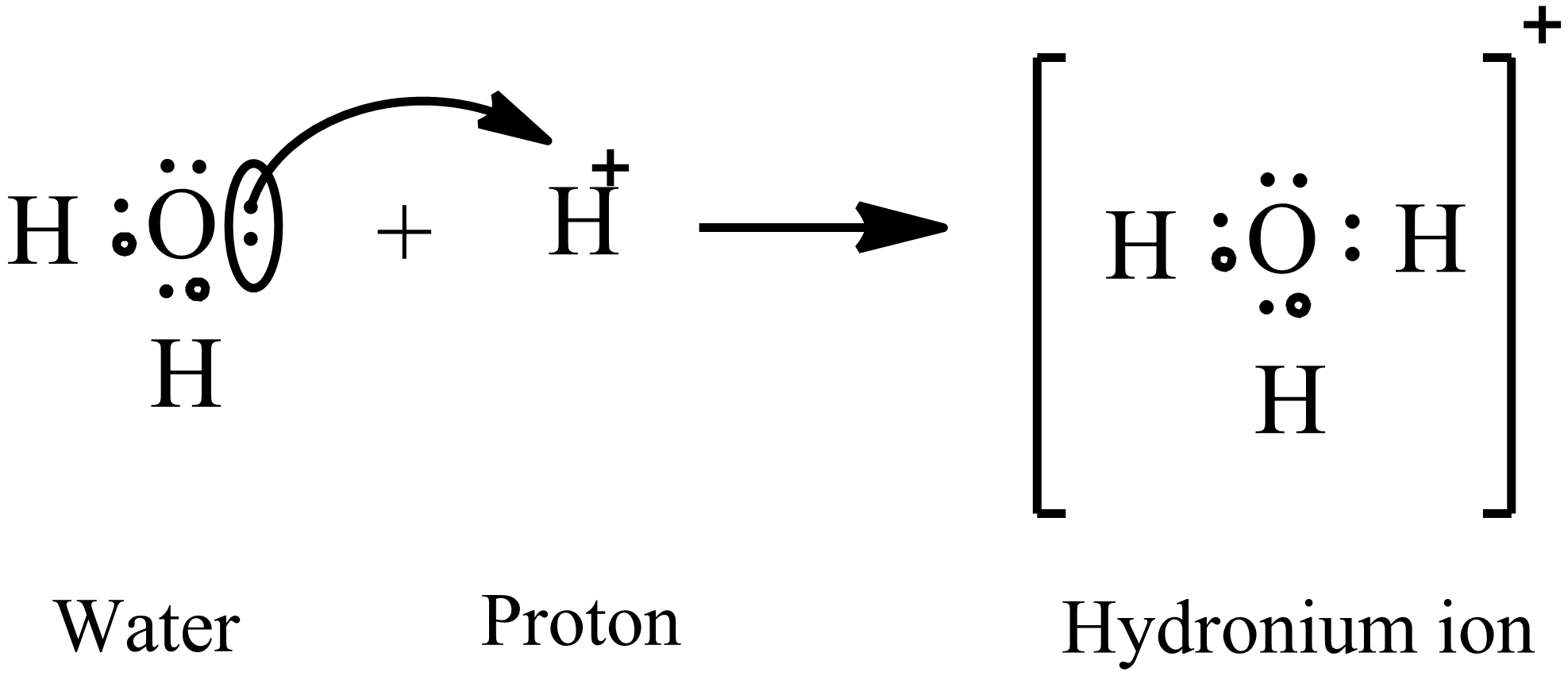


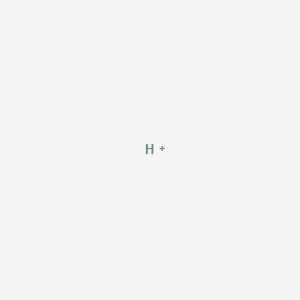










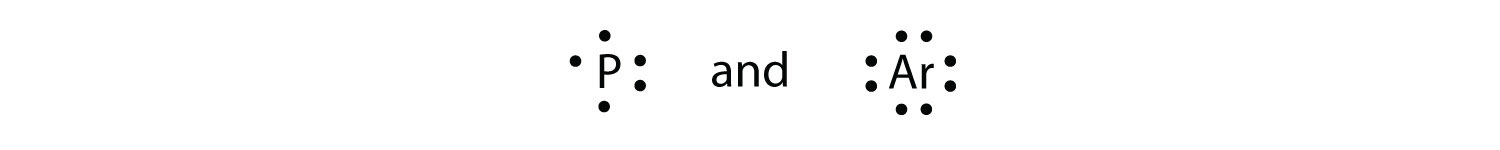













Comments
Post a Comment