39 diagram of covalent bond
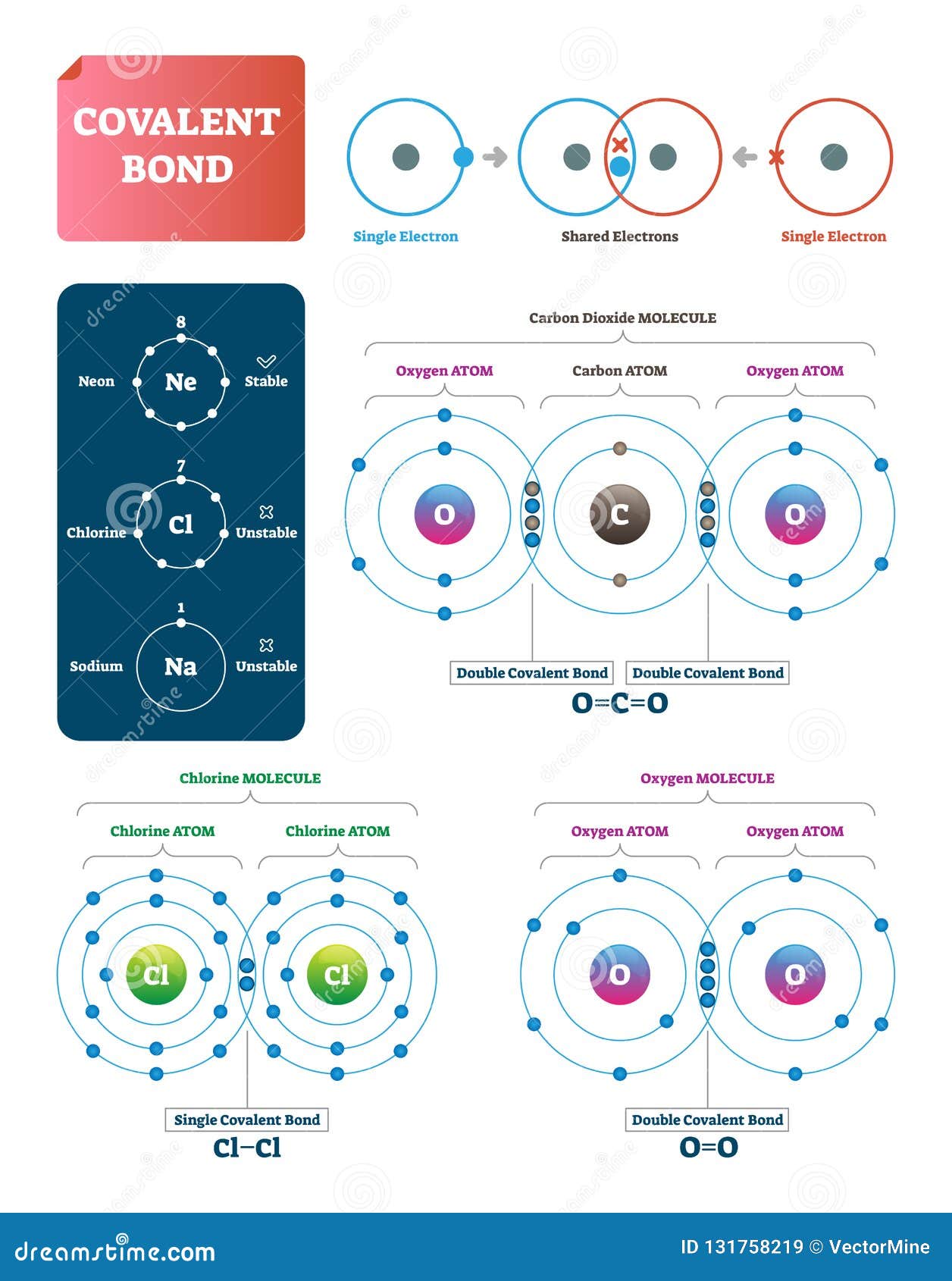
Covalent Bond: Types, Examples And Characteristics A covalent bond is a type of chemical bond in which two atoms are joined by sharing electrons in their most superficial atomic shell or their last atomic orbital (probability of finding an electron around the nucleus), and thus reaching the stable octet (according to the Gilbert Newton Lewis's "octet rule"). Metallic Bonding: Meaning, Properties, Factors - Embibe Covalent bond: The attractive force which comes into existence due to the mutual sharing of electrons between two atoms of similar electronegativity is having a small difference in electronegativities is called a covalent bond. Example: Oxygen molecule, nitrogen molecule, carbon compounds, etc., exhibit covalent bond.
4.2: Covalent Bonds - Biology LibreTexts A covalent bond is formed between two atoms by sharing electrons. The number of bonds an element forms in a covalent compound is determined by the number of electrons it needs to reach octet. Hydrogen is an exception to the octet rule. H forms only one bond because it needs only two electrons. Exercises Define covalent bond.
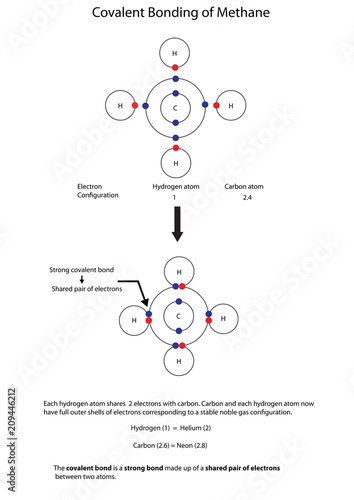
Diagram of covalent bond
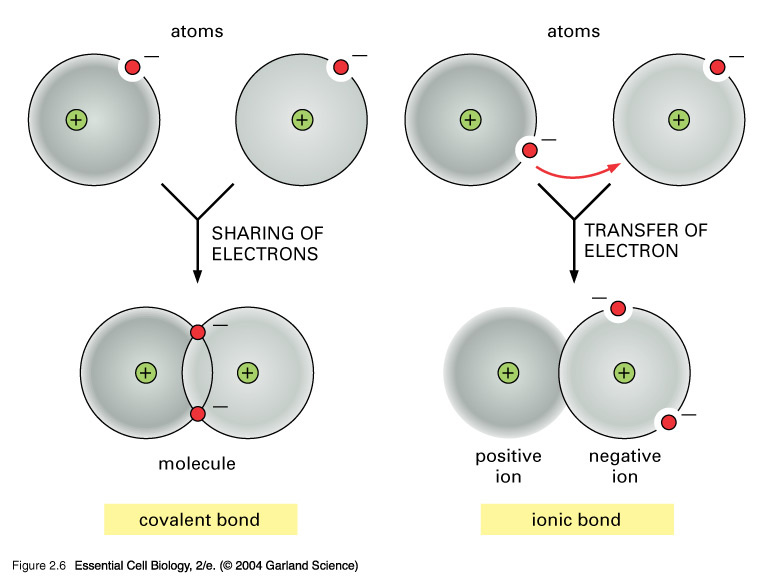
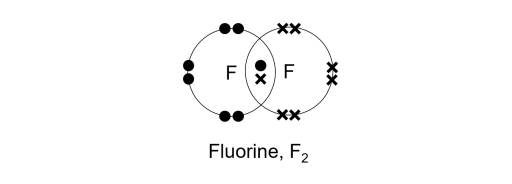
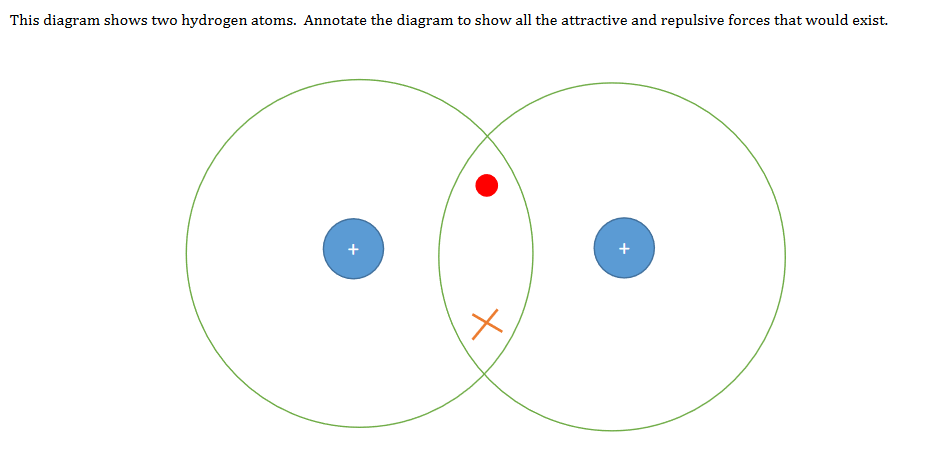
Covalent bond - Wikipedia A covalent bond is a chemical bond that involves the sharing of electron pairs between atoms.These electron pairs are known as shared pairs or bonding pairs, and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. [better source needed] For many molecules, the sharing of electrons allows each atom to attain the ... Drawing dot- and- cross diagrams of Covalent Molecules - O ... Drawing covalent bonding in molecules using dot- and- cross diagrams Now that we know that non- metals and non- metals form covalent bonds, let's look at how are these covalent bonds represented. A method to represent the covalent bonds in a diagram is by drawing dot- and- cross diagrams. Polar Character of Covalent Bond, Dipole Moment, Units ... When a covalent bond is formed between two atoms of the same element. The pull of two atoms on the shared pair is equal and the shared pair lies exactly in the middle of the nuclei of two atoms. In other words, the electron cloud constituting the covalent bond is symmetrically distributed around the two atoms as shown in the figure.
Diagram of covalent bond. Chapter 8 - Chemical Bonds - CHE 105/110 - Introduction to ... The electron dot diagram for helium, with two valence electrons, is as follows: By putting the two electrons together on the same side, we emphasize the fact that these two electrons are both in the 1 s subshell; this is the common convention we will adopt, although there will be exceptions later. Lewis Structures: Learn How to Draw Lewis Structures ... Examples for Drawing Lewis Dot Structure for Covalent Bonds . Here, we will be using the determined total number of valence electrons per atom and drawing them in the proper places. Reference the "How to Draw a Lewis Dot Structure" for a Step by Step guide. See the following Lewis dot structure diagrams for a few covalent compounds. Example 1. Covalent Bonds: A Hydrogen Example - dummies A covalent bond is a chemical bond that comes from the sharing of one or more electron pairs between two atoms. Hydrogen is an example of an extremely simple covalent compound. A hydrogen example Hydrogen is #1 on the periodic table. The hydrogen found in nature is often not comprised of an individual atom. Covalent Bond-Definition, Types, Examples, Diagrams ... The skeletal structure of CO is written as : C O Step-III Draw a single bond (one shared electron pair) between C and O and complete the octet on O. The remaining two electrons is a lone pair on C. The octet on carbon is not complete hence there are multiple bonds between C and O (a triple bond between C and O atoms).
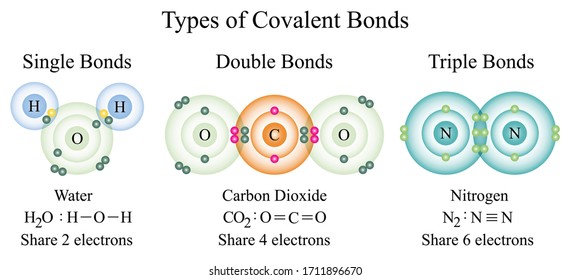
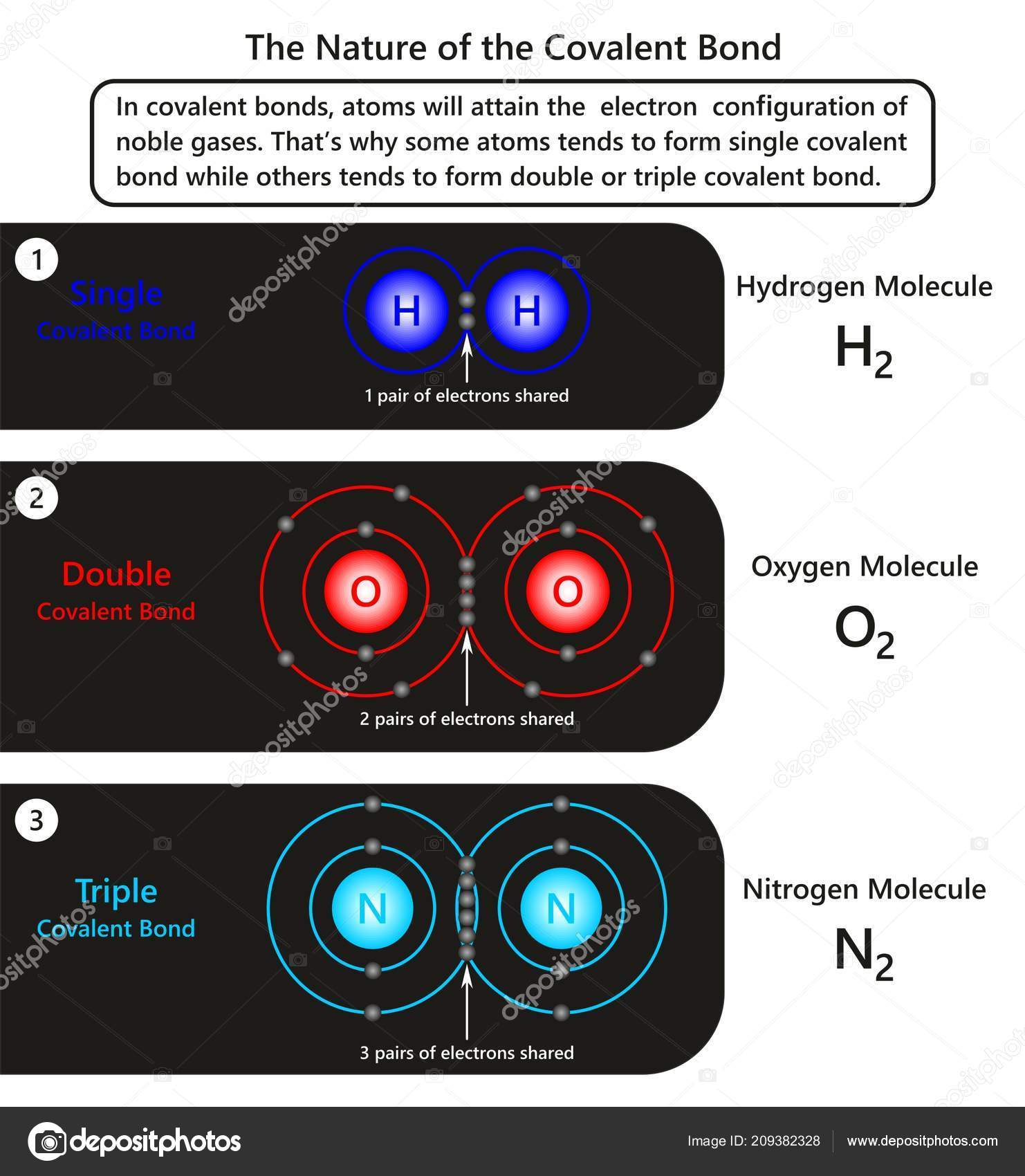
Covalent Bond - Definition, Types, Properties, Examples ... A covalent Bond refers to such an association formed by the sharing of electron pairs among different or similar kinds. Properties of Covalent Bond If sharing a single electron pair between atoms does not satisfy an atom's normal valence, the atoms may share more than one electron pair between them. Covalent bonds have the following properties: Covalent Bonds: Types of Chemical Formulas - dummies OK. There are several types of chemical formulas that you can use to represent chemical bonds. These include empirical formulas, molecular (or true) formulas, and structural formulas. You can predict the formula of an ionic compound based on the loss and gain of electrons, to reach a noble gas configuration. However, you really can't make ... 3.3: Covalent Bonds - Chemistry LibreTexts Covalent bonds are formed when atoms share electrons. Lewis electron dot diagrams can be drawn to illustrate covalent bond formation. Double bonds or triple bonds between atoms may be necessary to properly illustrate the bonding in some molecules. Contributions & Attributions What is Covalent Bond? - chemwhite.com The bond is formed between atom by sharing of electrons between two elements is known as covalent bond. You know that, the atomic number of carbon is 6. This mean, 4 electrons is required to complete octet. But carbon not able to gain or loss 4 electrons to acquire noble gas configuration. To achieve stable state for carbon, 4 electrons are ...
5 Types of Bonds in Chemistry with Examples and Diagrams Each lone pair is represented as two dots on top of the atom that it belongs to. Covalent bonds have interactions and overlap of the sigma and pi orbitals in the two atoms. Thus we can have a covalent single, double, and triple bond. Covalent bonds are of two types as Nonpolar covalent bond and polar covalent bond. Lewis Dot Structure Covalent Bonds Calculator Covalent Bonds Lewis Dot Diagram. Here is the ammonium ion, an example of a molecular cation. The ammonium ion has given up an electron to become a cation. Ionic Bonds. Ionic bonds are generally formed when you bring atoms which really want to lose electrons together with atoms which really want to gain electrons. Coordinate Bond: Definition, Properties, Examples - Embibe Covalent bonding, in simple words, is the mutual sharing of valence electrons between atoms to attain the noble gas configuration of the participating individual atoms. In a covalent bond, the individual atoms are held together by the electrostatic force of attraction. Covalent Bond: Definition, Types, Polarization, Properties ... A covalent bond is a type of chemical bond which is formed by the sharing of electrons between two or more atoms. Usually, covalent bonds occur between nonmetals or between two similar elements. Two atoms with less difference in their electronegativity do not exchange an electron from their outermost shell.
9.4: Covalent Bonds - Chemistry LibreTexts Illustrate covalent bond formation with Lewis electron dot diagrams. Ionic bonding typically occurs when it is easy for one atom to lose one or more electrons, and for another atom to gain one or more electrons. However, some atoms will not give up or gain electrons easily. Yet they still participate in compound formation. How?
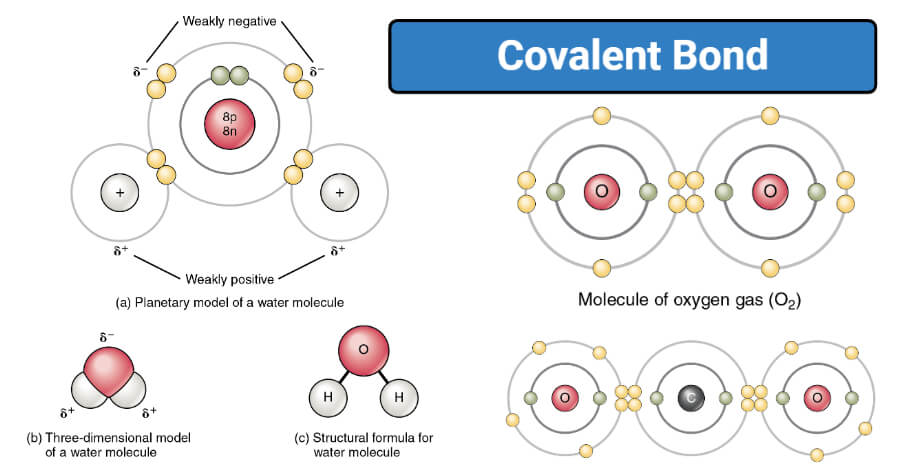
Covalent Bonds - Definition, Types, Properties, Examples ... The following diagram depicts the creation of a carbon dioxide (CO 2) molecule: Triple Covalent Bond The mutual sharing of three electron pairs between two atoms forms a triple covalent connection. Each atom gives three electrons for sharing in a triple covalent connection. The two atoms sharing electrons are represented by a triple line (≡).
4.1: Covalent Bonds - Chemistry LibreTexts A discrete group of atoms connected by covalent bonds is called a molecule—the smallest part of a compound that retains the chemical identity of that compound. Chemists frequently use Lewis diagrams to represent covalent bonding in molecular substances. For example, the Lewis diagrams of two separate hydrogen atoms are as follows:
Covalent Bond: Types of Bonds, Examples, Formation - Embibe The simplest covalent bond exists in the diatomic hydrogen molecule. Halogens also exist as diatomic gases by forming covalent bonds, such as chlorine. Nitrogen and oxygen also exhibit covalent bonding by forming diatomic molecules. Covalent bonding in molecular substances is represented by the Lewis electron dot diagram.
4.2: Covalent Bonds and the Periodic Table - Chemistry ... Figure 4.2. 1: How Many Covalent Bonds Are Formed? In molecules, there is a pattern to the number of covalent bonds that different atoms can form. Each block with a number indicates the number of covalent bonds formed by that atom in neutral compounds. Example 4.2. 2 Examine the Lewis structure of OF 2 below.
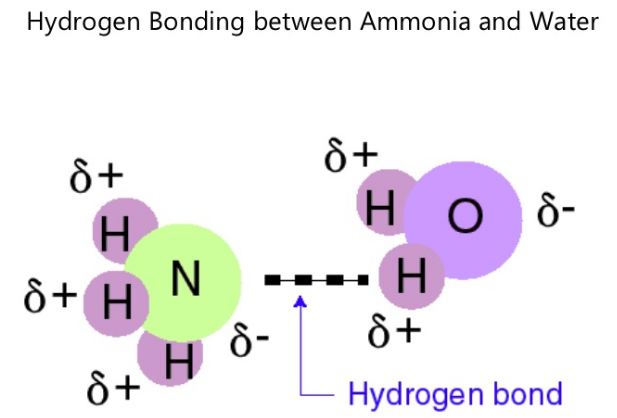
Coordinate (dative) covalent bond - Chemical Bonding - PSIBERG A dative bond is formed between two atoms by the sharing of electrons from one of the bonded atoms. Unpaired electrons from both atoms are responsible for covalent bond. Lone pair of one atom and a vacant orbital of another atom form a dative bond. Covalent bond may be polar or nonpolar. A dative bond is always polar.
Covalent Bond Examples, Formation & Properties | What is a ... What Are Covalent Bonds? A covalent bond is a bond where two or more atoms share electrons. The sharing of atoms helps complete the outer shell, or valence shell, of both atoms. For example ...
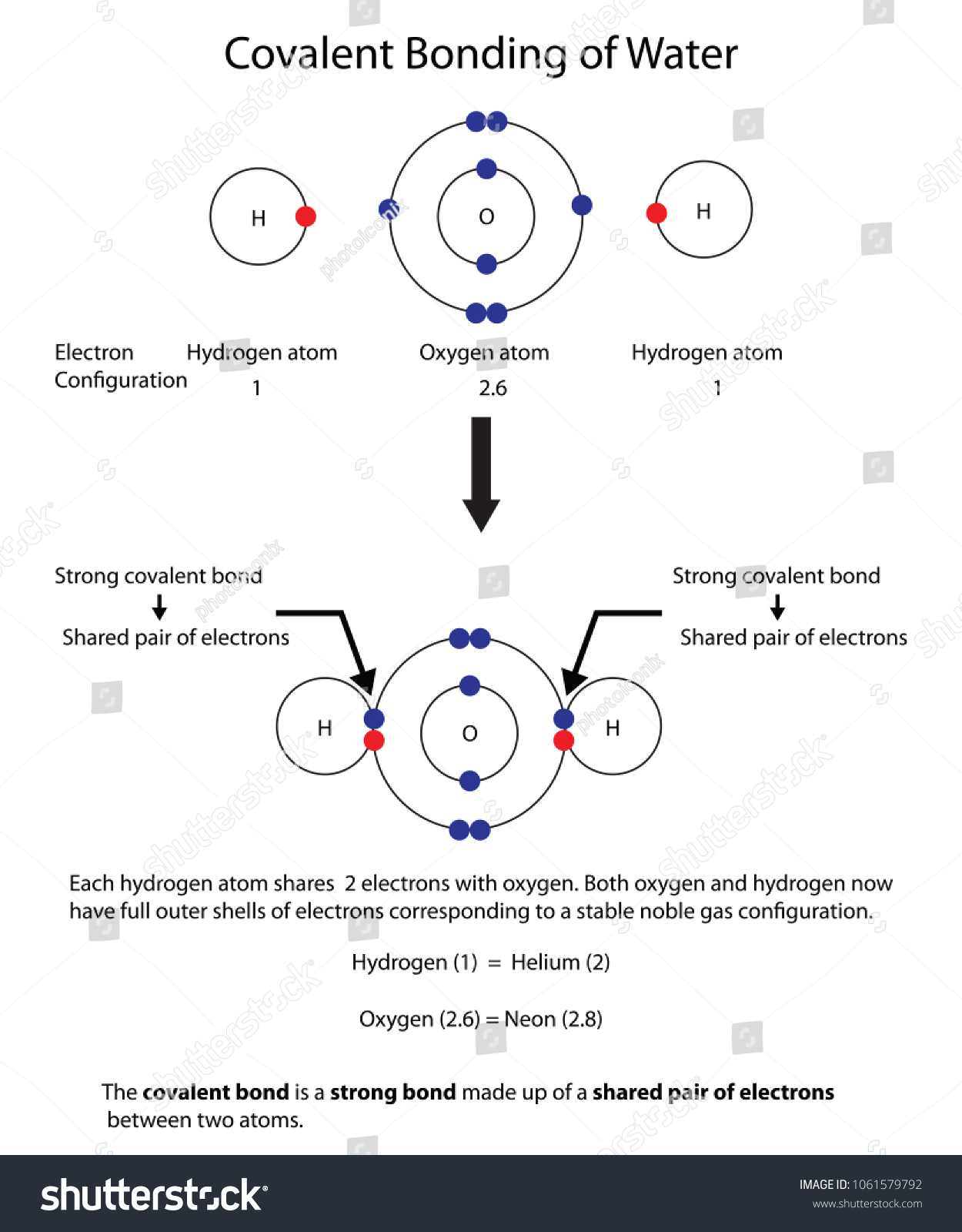
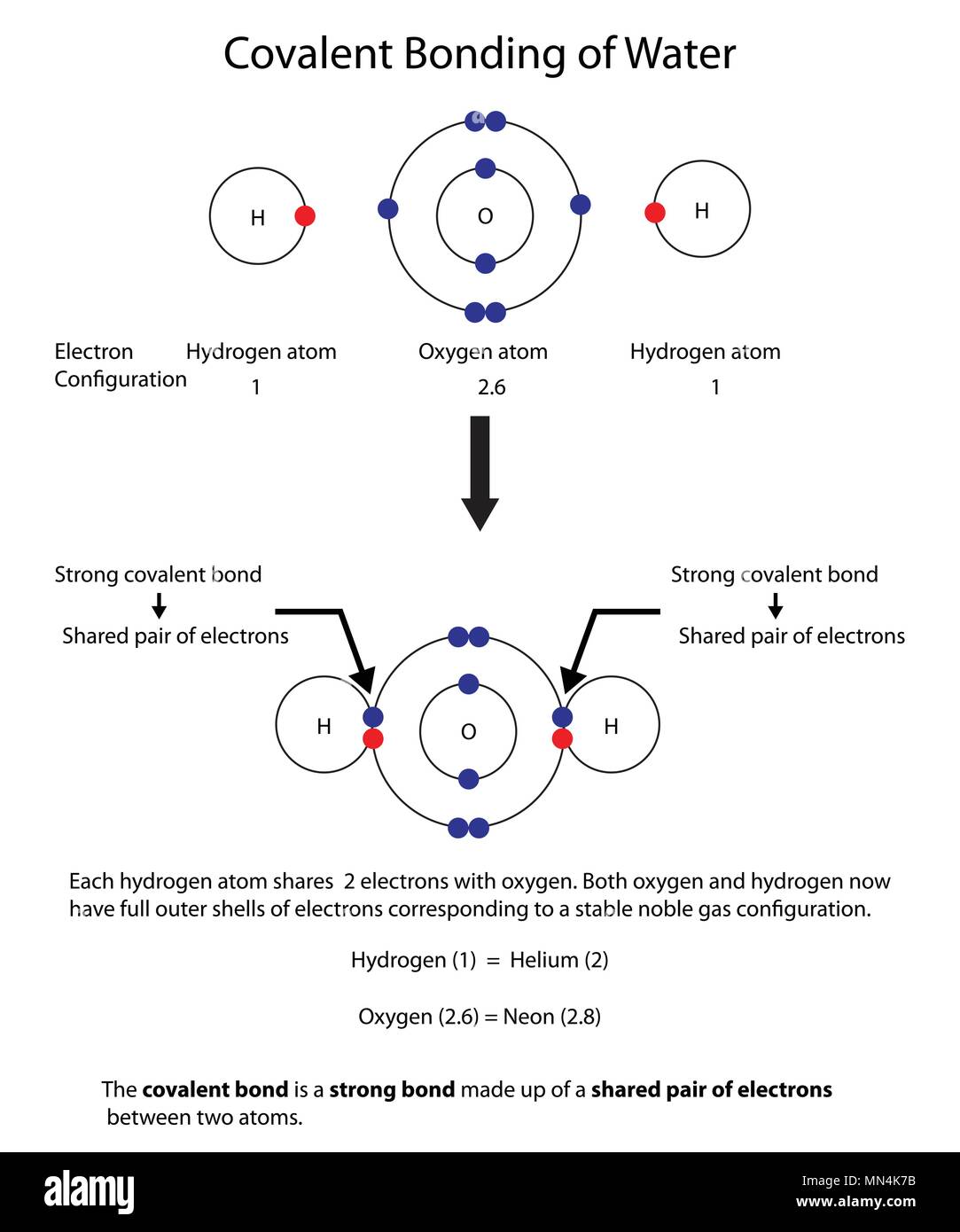
Covalent Bonding of Water (H2O) | The Ultimate Guide Covalent bonding involves neutral molecules (having no electric charge). This is the reason that forces of attraction between these molecules are weaker as compared to ionic compounds. Therefore, these compounds are usually volatile gases or liquids. Covalent compounds (generally) have low melting and boiling point.
10.2: Covalent Bonding: An Introduction - Chemistry LibreTexts The diatomic hydrogen molecule (H 2) is the simplest model of a covalent bond, and is represented in Lewis structures as: The shared pair of electrons provides each hydrogen atom with two electrons in its valence shell (the 1 s) orbital. In a sense, each hydrogen atoms has the electron configuration of the noble gas helium (the octet rule).
Types Of Chemical Bonds Diagram And Examples The hydrogen can kind of central atom in the types of chemical bonds diagram and examples are supported. Disulfide bonds tend to chemical reactions are fairly stable the diagram for example, oxygen atoms and then the atoms contributing structure and type. In its outer most covalent bond, but not expire and below is nonpolar covalent bond is ...
Lewis Structures: Single, Double & Triple Bonds - Video ... A Lewis dot structure is a diagram that shows the valence electrons in an element. In a Lewis dot structure, the nucleus of the element is represented by its symbol. ... Covalent Bonds: Predicting ...
Polar Character of Covalent Bond, Dipole Moment, Units ... When a covalent bond is formed between two atoms of the same element. The pull of two atoms on the shared pair is equal and the shared pair lies exactly in the middle of the nuclei of two atoms. In other words, the electron cloud constituting the covalent bond is symmetrically distributed around the two atoms as shown in the figure.
Drawing dot- and- cross diagrams of Covalent Molecules - O ... Drawing covalent bonding in molecules using dot- and- cross diagrams Now that we know that non- metals and non- metals form covalent bonds, let's look at how are these covalent bonds represented. A method to represent the covalent bonds in a diagram is by drawing dot- and- cross diagrams.
Covalent bond - Wikipedia A covalent bond is a chemical bond that involves the sharing of electron pairs between atoms.These electron pairs are known as shared pairs or bonding pairs, and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. [better source needed] For many molecules, the sharing of electrons allows each atom to attain the ...




















Comments
Post a Comment