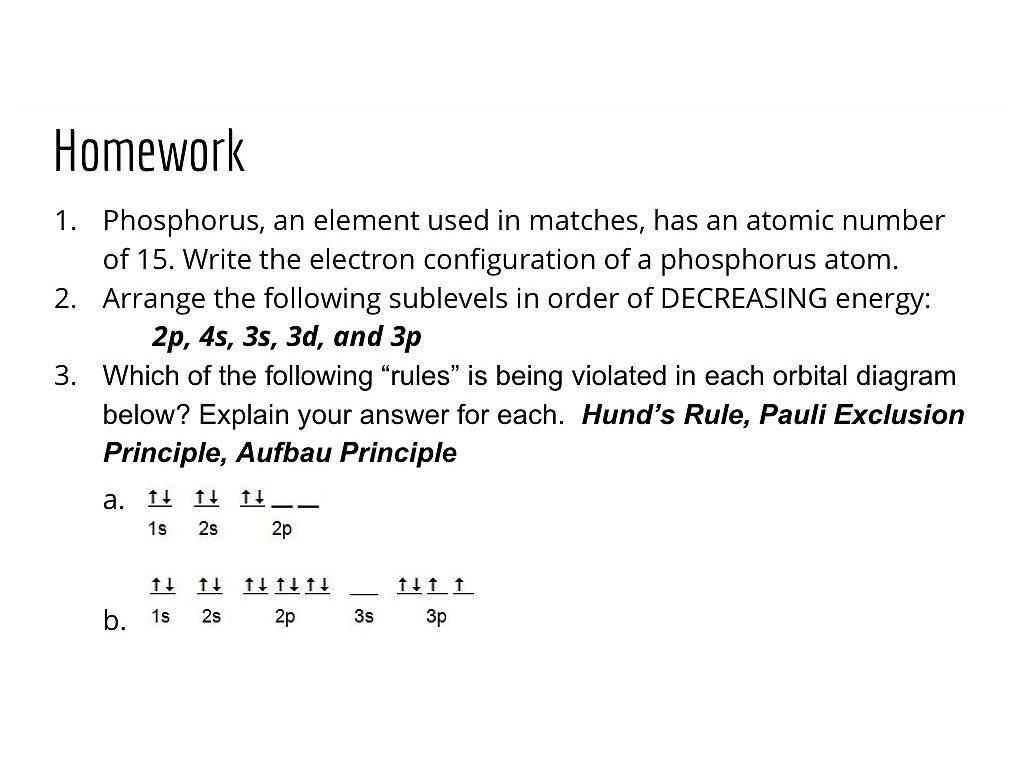
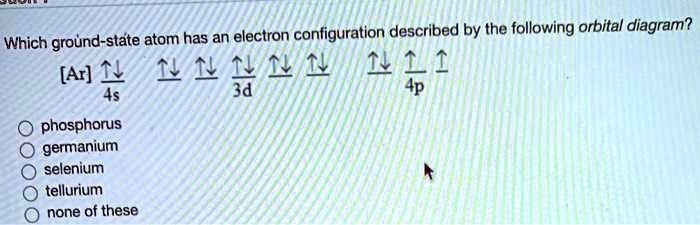
39 orbital diagram phosphorus
Draw and explain the orbital diagram for phosphorus ... Answer to: Draw and explain the orbital diagram for phosphorus. By signing up, you'll get thousands of step-by-step solutions to your homework... Phosphorus Electron Configuration (P) with Orbital Diagram 21 Jan 2021 — Phosphorous is a chemical element that has an atomic number of 15. Its electron configuration with regards to electrons present in each shell is ...
Aufbau Diagram For Phosphorus - schematron.org Electron Configurations and Orbital Diagrams KEY. Draw orbital diagrams for the following elements: 1. phosphorus. ↑↓. 1s. First, he Aufbau principle requires that lower energy orbitals are filled with Since phosphorus in a third period element, the first (K) and second (L) shells are .
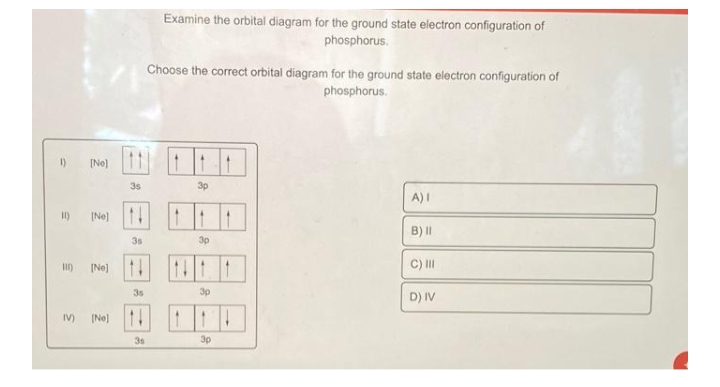
Orbital diagram phosphorus
Orbital Diagrams Flashcards - Quizlet What element is represented by this orbital diagram? P (Phosphorus) What element is represented by this orbital diagram? V (Vanadium) What element is represented by this orbital diagram? Pauli Exclusion Principle. 2 electrons in the same orbital must have opposite spins. Hund's Rule. How to Write the Orbital Diagram for Phosphorus (P) - YouTube To write the orbital diagram for the Phosphorus atom (P) first we need to write the electron configuration for just P. To do that we need to find the number... Quantum Numbers and Electron Configurations | Electronic ... The 15 electrons of the phosphorus atom will fill up to the 3 p orbital, which will contain three electrons: The last electron added is a 3 p electron. Therefore, n = 3 and, for a p -type orbital, l = 1. The ml value could be -1, 0, or +1. The three p orbitals are degenerate, so any of these ml values is correct.
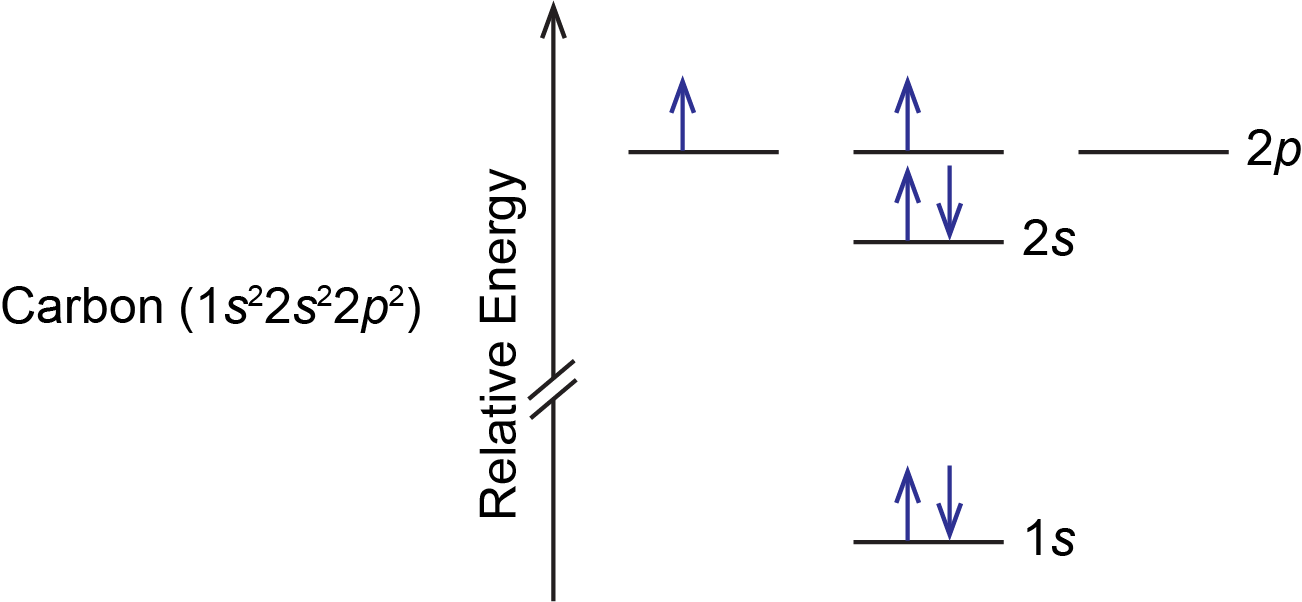
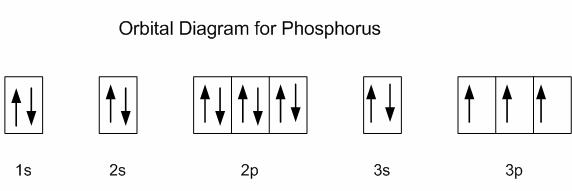
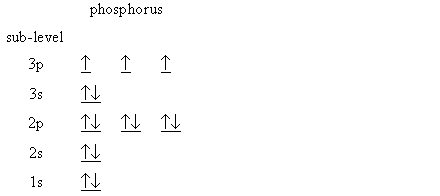
Orbital diagram phosphorus. Phosphorus Orbital diagram, Electron configuration, and ... The Phosphorus orbital diagram contains 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, the six electrons in the 2p orbital, the two electrons in the 3s orbital, and the remaining three electrons in the 3p orbital. The orbital diagram for a ground-state electron configuration of a Phosphorus atom is shown below- 1.4: Electron Configurations and Electronic Orbital Diagrams ... 30 May 2020 — The electron configuration for phosphorus is 1s2 2s2 2p 6 3s 2 3p 3 and the orbital diagram is drawn below. orbial diag phosphorus.png. 1.4: ... Electron configuration for Phosphorus (element 15 ... P (Phosphorus) is an element with position number 15 in the periodic table. Located in the III period. Melting point: 44 ℃. Density: 1.82 g/cm 3 . Electronic configuration of the Phosphorus atom: 1s 2 2s 2 2p 6 3s 2 3p 3. Reduced electronic configuration P: [Ne] 3s 2 3p 3. Below is the electronic diagram of the Phosphorus atom Distribution of ... Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. Free Gift for you: Interactive Periodic Table Let me tell you how this Interactive Periodic Table will help you in your studies. 1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table. 2).
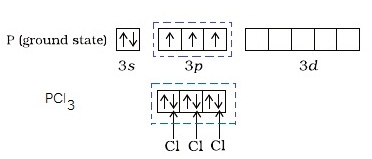
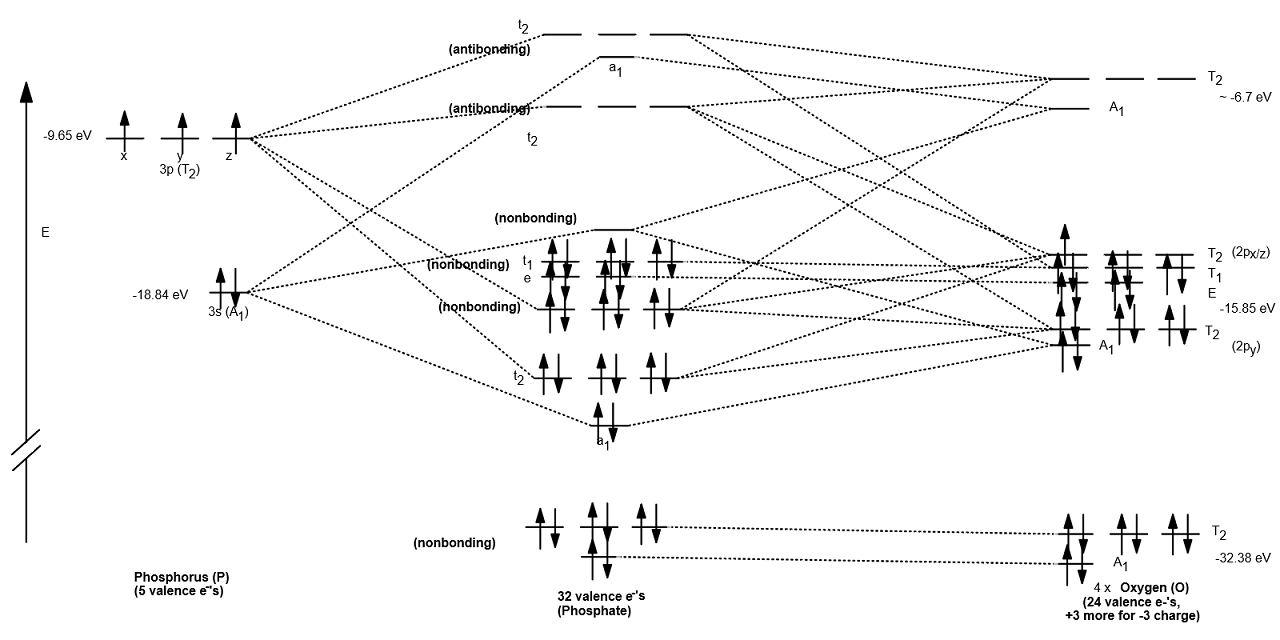
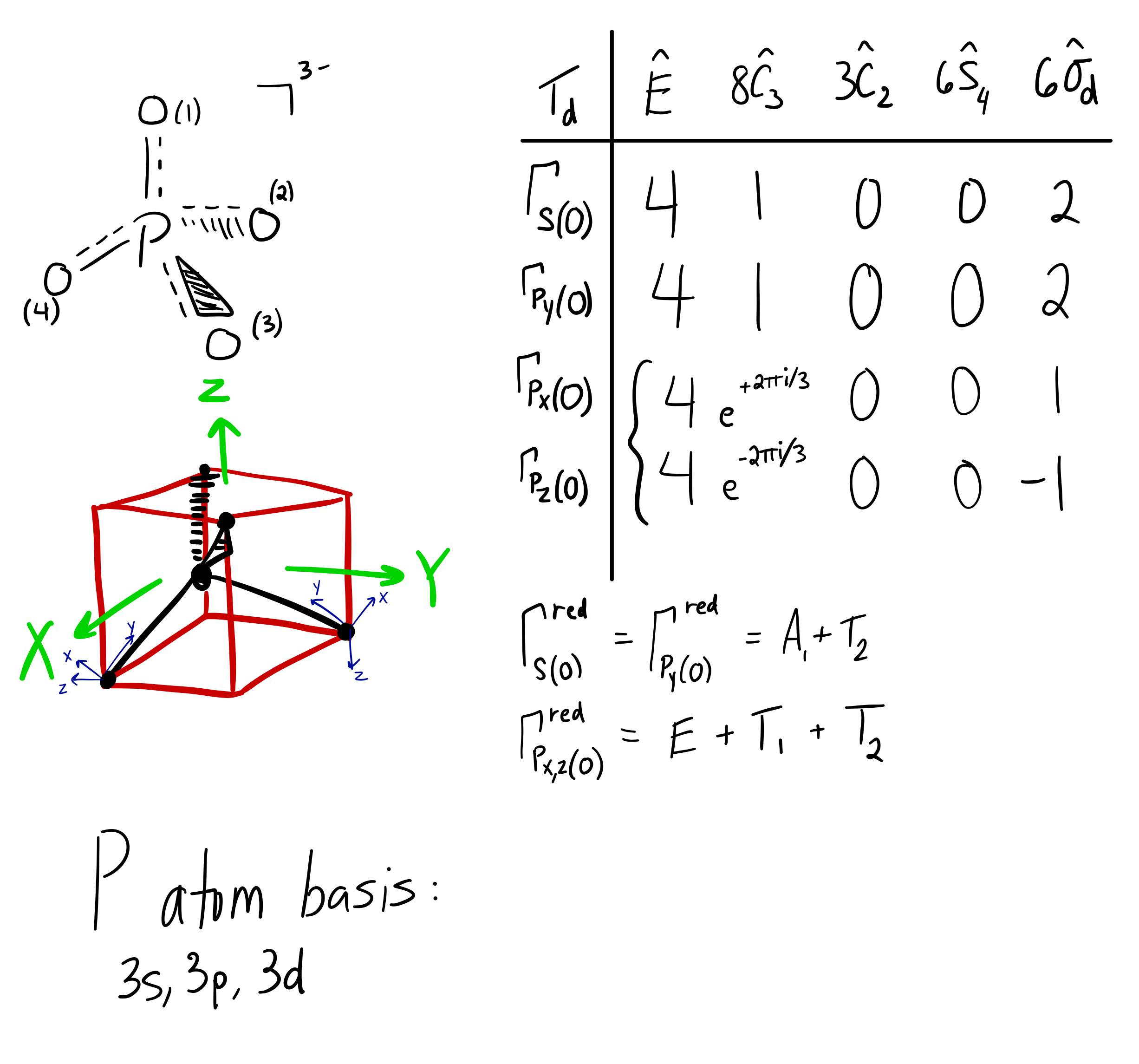
How do you write the orbital diagram for phosphate? | Socratic The whole point of that was to see how the oxygen orbital energies split up (and which were two-fold or three-fold degenerate). It was also to figure out which orbitals on phosphorus interact with which orbitals on the oxygen atoms. The resultant MO diagram was: Takeaways: This is only qualitative, so take it with a grain of salt. PDF Which of the following is the correct orbital diagram for ... The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2s and then 2p, 3s, and 3p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms. Solved Fill in the orbital energy diagram for phosphorus ... Chemistry. Chemistry questions and answers. Fill in the orbital energy diagram for phosphorus. 3p 2p. Solved What is the correct orbital diagram for phosphorus ... Science; Chemistry; Chemistry questions and answers; What is the correct orbital diagram for phosphorus? 1 2 3 4 5 What is the total capacity of electrons in n = 4? 5 ...
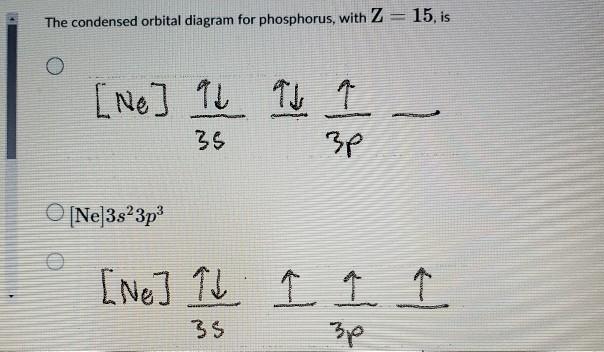
Aufbau Diagram For Phosphorus In writing the electron configuration for Phosphorus the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Phosphorous go in the 2s orbital. The next six electrons will go in the 2p orbital. PDF Electron Configurations and Orbital Diagrams key 2. The lobes of a p orbital disappear at the nucleus. What does this tell us about electrons in p orbitals? The probability of finding an electron at the nucleus is 0 (you will never find an electron in the nucleus). 3. 2The electron configuration for phosphorus, written in core notation, is [Ne] 3s 3p 3. What two things does Hund's rule tell us PLEASE HELP! Write the full electron ... - Brainly.com PLEASE HELP! Write the full electron configuration for phosphorus, atomic symbol P, then draw an orbital box diagram that accounts for all of the electrons in phosphorus. You don't need to include the orbital box diagram as part of your answer. Based on your drawing, explain why phosphorus is either paramagnetic or diamagnetic. Electron Configuration for Phosphorus (P) The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move to the 3p where we'll place the remaining three electrons. Therefore the Phosphorus electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 3. Video: Phosphorus Electron Configuration Notation
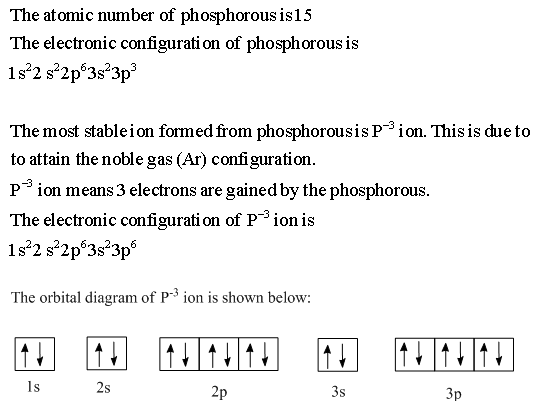
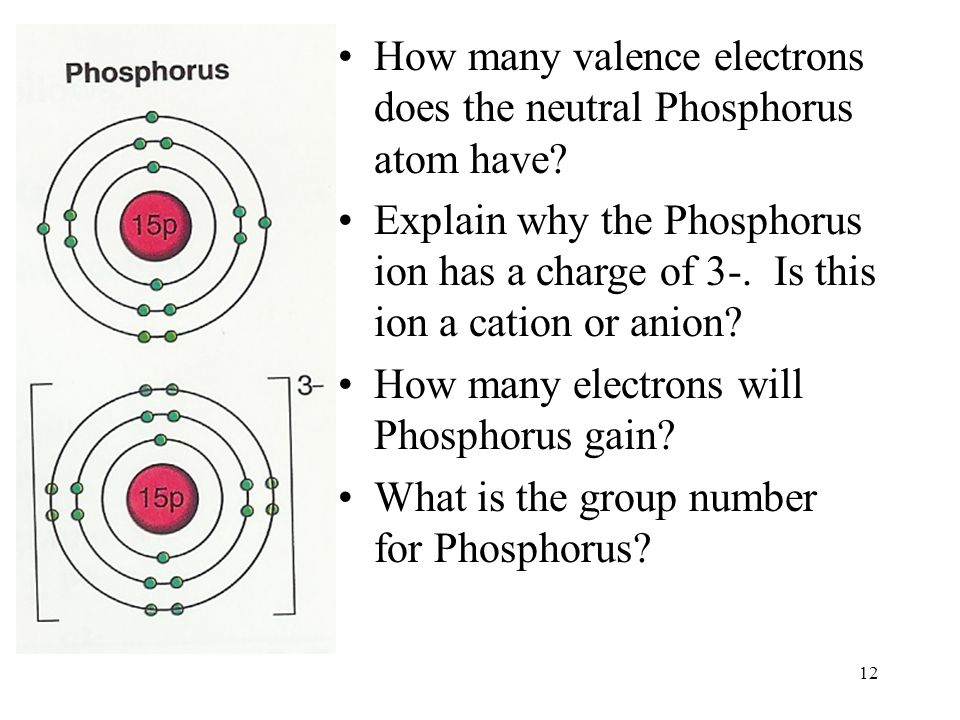
Orbital diagram phosphorus? - Answers Build the orbital diagram for the ion most likely formed by phosphorus? 1s22s22p63s23p3 is for Phosphorus and the most likely ion is to be a 3- because it wants to have a full outer shell therefore...
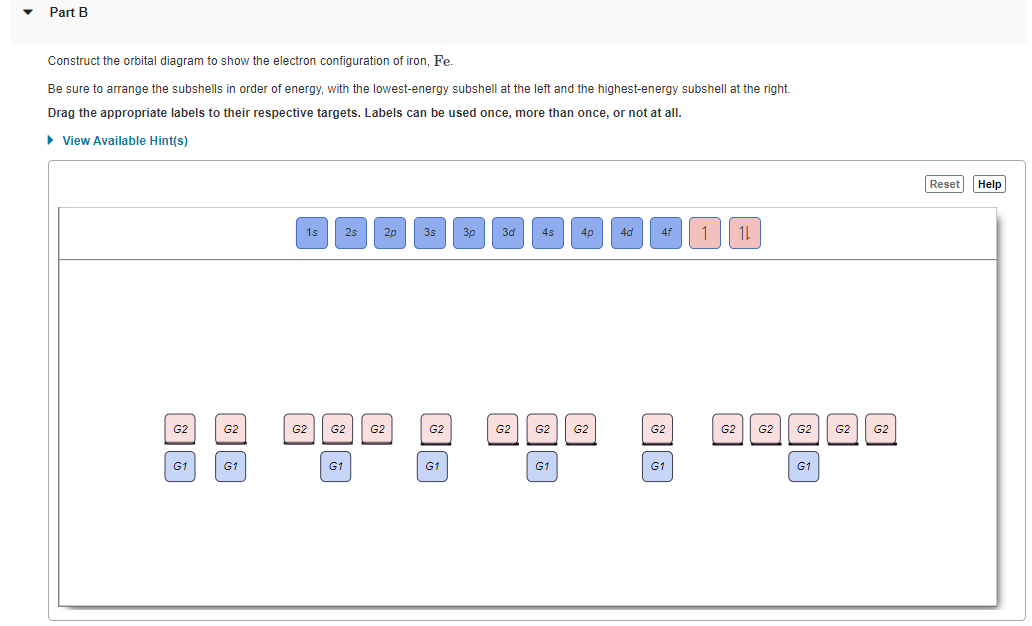
Build the orbital diagram for the ion most likely formed ... Build the orbital diagram for the ion most likely formed by phosphorus? Build the orbital diagram for the ion most likely formed by phosphorus. Does anyone know what it is and how to answer it on mastering chemistry, that **** is so confusing. for mastering chem make sure the lower numbers r at the bottum.
Orbital Box Diagram Phosphorus - schematron.org Feb 12, 2018 · A molecular orbital diagram or MO diagram for short is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the Linear combination of atomic orbitals molecular orbital method (LCAO method) in particular.schematron.org: Phosphorus: Orbital and Bonding InfoWhat is the orbital diagram of phosphorus
Phosphorus (P) - ChemicalAid Phosphorus (P) has an atomic mass of 15. Find out about its chemical and ... Orbital Diagram. P - Phosphorus - Orbital Diagram - Electron Configuration ...
What is the abbreviated electron configuration for phosphorus? Accordingly, what is the orbital diagram for phosphorus? The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move to the 3p where we'll place the remaining three electrons. Therefore the Phosphorus electron configuration will be 1s 2 2s 2 2p 6 ...
Build the orbital diagram for the ion most likely formed ... A blank molecular orbital diagram (Figure 2) has been provided to help you. chemistry what is the maximum number of grams of PH3 that can be formed when 6.2 g of phosphorus reacts with 4.0 g of hydrogen to form PH3
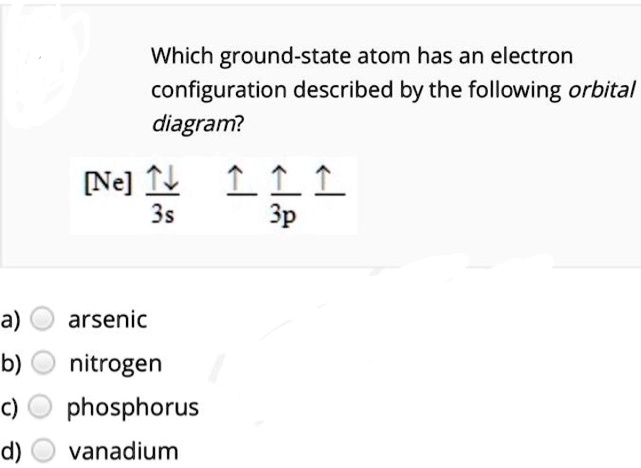
Answered: How many unpaired electrons are in the… | bartleby Science Chemistry Q&A Library How many unpaired electrons are in the orbital diagram for phosphorus? Group of answer choices A) 0 B) 6 C) 2 D) 3 E) 1. How many unpaired electrons are in the orbital diagram for phosphorus? Group of answer choices A) 0 B) 6 C) 2 D) 3 E) 1. Question.
What is the electron configuration for an ... - Socratic.org Phosphorus has atomic number 15. As such its electronic configuration in ground state is 1s^2 2s^2 2p^6 3s^2 3p^3 The following electronic configurations could be excited states 1s^2 2s^2 2p^6 3s^1 3p^4 Here one of 3s electrons has been promoted to 3p sub level 1s^2 2s^2 2p^6 3s^2 3p^2 3d^1 Here one of 3p electrons has been promoted to 3d sub level 1s^2 2s^2 2p^6 3s^2 3p^2 4s^1 Another ...
What is the orbital diagram for phosphorus? | Study.com What is the orbital diagram for phosphorus? Orbital Diagrams: Orbital diagrams show the distribution of electrons in the electron shells and subshells of an atom. A few principles and rules must be...
The orbital diagram of ground state Phosphorus atom has to ... The orbital diagram of ground state Phosphorus atom has to be written. Concept introduction: Pauli Exclusion Principle An orbital having a most two electrons and in this two electrons have opposite spins. Each orbital having no more than two electrons and similar spin is not allowed.
Phosphorus(P) electron configuration and orbital diagram Orbital diagram for phosphorus (P) Electron configuration of phosphorus in the excited state Atoms can jump from one orbital to another in the excited state. This is called quantum jump. The ground state electron configuration of phosphorus is 1s 2 2s 2 2p 6 3s 2 3p 3. The p-orbital has three sub-orbitals. The sub-orbitals are p x, p y, and p z.
Quantum Numbers and Electron Configurations | Electronic ... The 15 electrons of the phosphorus atom will fill up to the 3 p orbital, which will contain three electrons: The last electron added is a 3 p electron. Therefore, n = 3 and, for a p -type orbital, l = 1. The ml value could be -1, 0, or +1. The three p orbitals are degenerate, so any of these ml values is correct.
How to Write the Orbital Diagram for Phosphorus (P) - YouTube To write the orbital diagram for the Phosphorus atom (P) first we need to write the electron configuration for just P. To do that we need to find the number...
Orbital Diagrams Flashcards - Quizlet What element is represented by this orbital diagram? P (Phosphorus) What element is represented by this orbital diagram? V (Vanadium) What element is represented by this orbital diagram? Pauli Exclusion Principle. 2 electrons in the same orbital must have opposite spins. Hund's Rule.











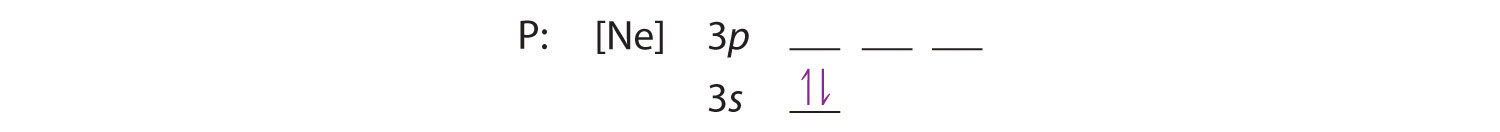




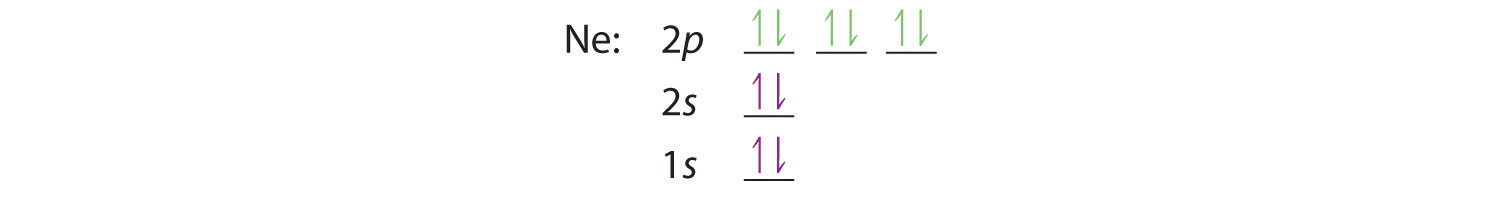




Comments
Post a Comment